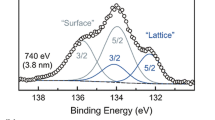
Abstract
Solar thermochemical hydrogen (STCH) production from water splitting typically requires performing redox cycles at temperatures above 1200 °C to reduce and re-oxidize the bulk of a reversible material. Bulk processes such as oxygen vacancy formation and oxygen diffusion energies dictate the viability of a material for STCH. The surface plays an important role in the formation and destruction of vacancies and interacts with gas phase water and surface adsorbed species. These surface processes can lead to surface reconfigurations and even the formation of surface phases with stoichiometry and oxygen content very different from the bulk composition. Understanding in-situ the surface chemical state and its evolution under water splitting is important to design nonstoichiometric oxides capable of longer-lasting STCH generation at lower temperatures. In this work, we describe the water splitting active defect sites in LSM ((La0.65Sr0.35)0.95MnO3−δ) and Ga-doped LSM ((La0.6Sr0.4)0.95(Mn0.8Ga0.2)O3−δ) perovskites during Operando thermochemical water splitting conditions using ambient-pressure X-ray photoelectron spectroscopy (AP-XPS) experiments at 800 °C under steam. We show that sub-stoichiometric La+3 in the oxygen-vacancy rich surface at operating conditions can be used to correlate surface water splitting activity and the creation of surface hydroxide intermediates. The addition of Ga in LSM is shown to drastically stabilize the surface chemical composition by preventing Sr segregation and stabilizing catalytically active surface defects that promote the binding of adsorbed hydroxides. We use Operando AP-XPS quantification of metastable surface hydroxide intermediates (La(OH)3) to determine the amount of catalytically active surface sites in LSM (2.9%) and in LSMG (7.8–8.1%, depending on the bulk oxidation state).






Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Bergenti I, Dediu V, Arisi E, Cavallini M, Biscarini F, Taliani C, de Jong MP, Dennis CL, Gregg JF, Solzi M, Natali M (2007) Spin polarized La0.7Sr0.3MnO3 thin films on silicon. J Magn Magn Mater 312(2):453–457. https://doi.org/10.1016/j.jmmm.2006.11.221
Gokon N, Hara K, Sugiyama Y, Bellan S, Kodama T, Hyun-seok C (2019) Thermochemical two-step water splitting cycle using perovskite oxides based on LaSrMnO3 redox system for solar H2 production. Thermochim Acta 680:178374. https://doi.org/10.1016/j.tca.2019.178374
McDaniel AH, Miller EC, Arifin D, Ambrosini A, Coker EN, O’Hayre R, Chueh WC, Tong J (2013) Sr- and Mn-doped LaAlO3−δ for solar thermochemical H2 and CO production. Energy Environ Sci 6(8):2424–2428. https://doi.org/10.1039/C3EE41372A
Song H, Luo S, Huang H, Deng B, Ye J (2022) Solar-driven hydrogen production: recent advances, challenges, and future perspectives. ACS Energy Lett 7(3):1043–1065. https://doi.org/10.1021/acsenergylett.1c02591
Koo B, Kim K, Kim JK, Kwon H, Han JW, Jung W (2018) Sr segregation in perovskite oxides: why it happens and how it exists. Joule 2(8):1476–1499. https://doi.org/10.1016/j.joule.2018.07.016
Scheffe JR, Weibel D, Steinfeld A (2013) Lanthanum–strontium–manganese perovskites as redox materials for solar thermochemical splitting of H2O and CO2. Energy Fuels 27(8):4250–4257. https://doi.org/10.1021/ef301923h
Cai Z, Kubicek M, Fleig J, Yildiz B (2012) Chemical heterogeneities on La0.6Sr0.4CoO3−δ thin films—correlations to cathode surface activity and stability. Chem Mater 24(6):1116–1127. https://doi.org/10.1021/cm203501u
Bliem R, Kim D, Wang J, Crumlin EJ, Yildiz B (2021) Hf deposition stabilizes the surface chemistry of perovskite manganite oxide. J Phys Chem C 125(6):3346–3354. https://doi.org/10.1021/acs.jpcc.0c09707
Li H, Kang H-S, Liu Z, Barkhordarian O, Lee S, Yoon Y, Lee MH (2022) Effect of angstrom-level oxide overcoat on Sr segregation behavior of LSM electrodes. Int J Hydrogen Energy 47(77):33058–33066. https://doi.org/10.1016/j.ijhydene.2022.07.200
Scheffe JR, McDaniel AH, Allendorf MD, Weimer AW (2013) Kinetics and mechanism of solar-thermochemical H2 production by oxidation of a cobalt ferrite–zirconia composite. Energy Environ Sci 6(3):963–973. https://doi.org/10.1039/C3EE23568H
Barcellos DR, Sanders MD, Tong J, McDaniel AH, O’Hayre RP (2018) BaCe0.25Mn0.75O3−δ—a promising perovskite-type oxide for solar thermochemical hydrogen production. Energy Environ Sci 11(11):3256–3265. https://doi.org/10.1039/C8EE01989D
Wu Q-H, Liu M, Jaegermann W (2005) X-ray photoelectron spectroscopy of La0.5Sr0.5MnO3. Mater Lett 59(16):1980–1983. https://doi.org/10.1016/j.matlet.2005.01.038
Chaluvadi SK, Polewczyk V, Petrov AY, Vinai G, Braglia L, Diez JM, Pierron V, Perna P, Mechin L, Torelli P, Orgiani P (2022) Electronic properties of fully strained La1–xSrxMnO3 thin films grown by molecular beam epitaxy (0.15 ≤ x ≤ 0.45). ACS Omega 7(17):14571–14578. https://doi.org/10.1021/acsomega.1c06529
Jalili H, Chen Y, Yildiz B (2010) Structural, chemical, and electronic state on La0.7Sr0.3MnO3 dense thin-film surfaces at high temperature: surface segregation. ECS Trans 28(11):235. https://doi.org/10.1149/1.3495846
Hishida T, Ohbayashi K, Kobata M, Ikenaga E, Sugiyama T, Kobayashi K, Okawa M, Saitoh T (2013) Empirical relationship between x-ray photoemission spectra and electrical conductivity in a colossal magnetoresistive manganite La1−xSrxMnO3. J Appl Phys. https://doi.org/10.1063/1.4811372
Chueh WC, McDaniel AH, Grass ME, Hao Y, Jabeen N, Liu Z, Haile SM, McCarty KF, Bluhm H, El Gabaly F (2012) Highly enhanced concentration and stability of reactive Ce3+ on doped CeO2 surface revealed in operando. Chem Mater 24(10):1876–1882. https://doi.org/10.1021/cm300574v
Lee HB, Prinz FB, Cai W (2010) Atomistic simulations of surface segregation of defects in solid oxide electrolytes. Acta Mater 58(6):2197–2206. https://doi.org/10.1016/j.actamat.2009.12.005
Choi M, Ibrahim IAM, Kim K, Koo JY, Kim SJ, Son J-W, Han JW, Lee W (2020) Engineering of charged defects at perovskite oxide surfaces for exceptionally stable solid oxide fuel cell electrodes. ACS Appl Mater Interfaces 12(19):21494–21504. https://doi.org/10.1021/acsami.9b21919
Lee W, Han JW, Chen Y, Cai Z, Yildiz B (2013) Cation size mismatch and charge interactions drive dopant segregation at the surfaces of manganite perovskites. J Am Chem Soc 135(21):7909–7925. https://doi.org/10.1021/ja3125349
Miller JE, McDaniel AH, Allendorf MD (2014) Considerations in the design of materials for solar-driven fuel production using metal-oxide thermochemical cycles. Adv Energy Mater 4(2):19. https://doi.org/10.1002/aenm.201300469
McCord DC, Gager EJ, Lee K, McDaniel AH, Nino JC, Scheffe JR (2023) Assessment of bulk oxygen capacity and transient redox behavior of foamed lanthanum strontium manganese perovskites. J Solar Energy Eng. https://doi.org/10.1115/1.4063440
Wang X, Huang K, Yuan L, Xi S, Yan W, Geng Z, Cong Y, Sun Y, Tan H, Wu X, Li L, Feng S (2018) Activation of surface oxygen sites in a cobalt-based perovskite model catalyst for CO oxidation. J Phys Chem Lett 9(15):4146–4154. https://doi.org/10.1021/acs.jpclett.8b01623
Ponce S, Peña MA, Fierro JLG (2000) Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Appl Catal B 24(3):193–205. https://doi.org/10.1016/S0926-3373(99)00111-3
Sunding MF, Hadidi K, Diplas S, Løvvik OM, Norby TE, Gunnæs AE (2011) XPS characterisation of in situ treated lanthanum oxide and hydroxide using tailored charge referencing and peak fitting procedures. J Electron Spectrosc Relat Phenom 184(7):399–409. https://doi.org/10.1016/j.elspec.2011.04.002
Mickevičius S, Grebinskij S, Bondarenka V, Lisauskas V, Šliužienė K, Tvardauskas H, Vengalis B, Orlowski BA, Osinniy V, Drube W (2009) The surface hydro-oxidation of LaNiO3−δ thin films. Micron 40(1):135–139. https://doi.org/10.1016/j.micron.2008.01.006
Siegmann HC, Schlapbach L, Brundle CR (1978) Self-restoring of the active surface in the hydrogen sponge LaNi5. Phys Rev Lett 40(14):972–975. https://doi.org/10.1103/PhysRevLett.40.972
Li JPH, Zhou X, Pang Y, Zhu L, Vovk EI, Cong L, van Bavel AP, Li S, Yang Y (2019) Understanding of binding energy calibration in XPS of lanthanum oxide by in situ treatment. Phys Chem Chem Phys 21(40):22351–22358. https://doi.org/10.1039/C9CP04187G
Zhao Z, Ling X, El Gabaly F, Grass M, Jabeen N, Jones D, Liu Z, Mun BS, Braun A, Chen Q (2022) Observation of potential-induced hydration on the surface of ceramic proton conductors using in situ near-ambient pressure X-ray photoelectron spectroscopy. J Phys Chem Lett 13(13):2928–2933. https://doi.org/10.1021/acs.jpclett.2c00114
Acknowledgements
We gratefully acknowledge financial support from the U.S. Department of Energy Hydrogen and Fuel Cell Technologies Office (Award Number: DE-EE0008840). Sandia National Laboratories is a multi-mission laboratory managed and operated by National Technology and Engineering Solutions of Sandia, LLC (NTESS), a wholly owned subsidiary of Honeywell International Inc., for the U.S. Department of Energy’s National Nuclear Security Administration (DOE/NNSA) under contract DE-NA0003525. This written work is authored by an employee of NTESS. The employee, not NTESS, owns the right, title and interest in and to the written work and is responsible for its contents. This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the written work do not necessarily represent the views of the U.S. Government. The publisher acknowledges that the U.S. Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this written work or allow others to do so, for U.S. Government purposes. The DOE will provide public access to results of federally sponsored research in accordance with the DOE Public Access Plan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernandes Cauduro, A.L., Gager, E., King, K.A. et al. Stabilization of Catalytically Active Surface Defects on Ga-doped La–Sr–Mn Perovskites for Improved Solar Thermochemical Generation of Hydrogen. Top Catal (2024). https://doi.org/10.1007/s11244-024-01940-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11244-024-01940-w