Abstract
We discuss the similarities and differences between catalysis/electrocatalysis of chemical processes, such as ammonia synthesis or H2O electrolysis on one hand, and of nuclear catalytic processes, commonly called baryosynthesis, such as the synthesis of neutrons and protons from quarks, on the other. In chemical synthesis the underlying forces are well known to be electrostatic in nature while in the synthesis of hadrons or nuclei from quarks (known as hadronization or nucleosynthesis) the underlying forces are known as the Strong nuclear forces, whereas if electrons are also involved, as Weak nuclear forces. Here we discuss for the first time from a catalytic viewpoint the importance of some recent developments in our understanding of the structure and synthesis of hadrons via a model entitled Rotating Lepton Model (RLM), which is quite similar to the Bohr model of the H atom used in Chemistry but which has shown that the Strong Force is a gravitational force between three very fast (relativistic) neutrinos, rotating symmetrically on a circular orbit, whose gravitational masses and gravitational attraction increases dramatically with increasing rotational speed, according to the theory of Special Relativity (SR), thus reaching the masses of quarks and the value of the Strong Force respectively. We show that, interestingly, positrons and electrons, which quite often play a very important and well established catalytic role in chemical synthesis due to their electrical charge, also play an equally important and central catalytic role in nuclear synthesis due to their enormous mass, relative to the mass of the neutrinos, and the concomitant dramatic acceleration of neutrinos to ultrarelativistic speeds and huge mass increase, resulting to enhanced very strong gravitational binding between them which reaches the value of the Strong Force. Consequently, electrons and positrons are the dual, electrostatic and gravitational, catalysts of our Universe for the production of chemicals and baryons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The paramount importance of Catalysis and Electrocatalysis in Chemistry but also in Biology for the synthesis of chemical and biochemical products is to a significant extent well known and understood at the molecular level [1,2,3,4,5,6,7,8].
For example, it is common knowledge that the synthesis of ammonia or the production of oxygen can only be accomplished efficiently via the use of efficient catalysts and electrocatalysts respectively.
On the other hand very little is known about the catalysis of nuclear reactions for the synthesis of composite particles such as protons and neutrons (more generally of hadrons) from their constituents which are currently believed, according to the Standard Model (SM) of elementary particles [9], to be quarks, which are still widely considered to be the elementary constituents of matter, although so far they have never been isolated and studied. Under such, so far relatively obscure, conditions it is very hard to examine within the context of the SM the factors which may catalyze the combination of quarks to form hadrons (such as protons and neutrons) or, vice versa, hadron decomposition.
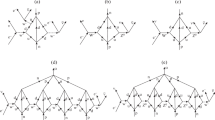
However, the recent development, as a promising alternative to the Standard Model (SM), of the Rotating Lepton Model (RLM), [10,11,12,13,14,15], (Fig. 1), which bears some interesting similarities with the Bohr model of the H atom [2], (Table 1), and sheds some new light on the nature of the reactants, of the dominant confining force and of the mechanism of hadronization. These considerations have been first explored in a paper published in 2017, entitled “Catalysis and Electrocatalysis of Chemical Synthesis and of Hadronization” [15]. In that study, some of the key catalytic points discussed here, had been already identified. During the last five years, however, several new facts have emerged regarding the mechanism of hadronization and the fundamental role of positrons and electrons in the hadronization process, i.e. in the generation of hadrons, such as protons and neutrons which merit some discussion.
Rotating Lepton Model (RLM) for the formation of a neutron from three rotating neutrinos. It comprises the equation of motion (1) and the De Broglie Eq. (2) for each rotating neutrino, and also the energy balance Eq. (3) for computing the energy E and the mass 3γmo of the rotating triad, where γ(= (1-v2/c2)−1/2) is the Lorentz factor
Detailed careful examination of the decay products of composite particles [16] shows that there exist only five elementary particles in our Universe, i.e. electrons, positrons and the three types of neutrinos [10,11,12,13,14]. All composite particles eventually decay to them.
2 Results and Discussion
2.1 Thermodynamic Similarities and Equilibrium Conversion
The basic fundamental similarity between chemical synthesis (e.g. ammonia or methanol synthesis) and hadronization (e.g. formation of baryons from quarks) is that both are highly exothermic processes as shown in Table 2. In chemical synthesis the internal energy change, ΔU, of the synthesis reaction is of the order of -2 eV per atom, while in hydrogen fusion it is near -26 MeV per He nucleus produced, and in baryonsynthesis it is of the order of -0.15 to -1.5 GeV per baryon formed. Thus the adiabatic temperatures of these reactions vary between 103 and 104 K for chemical synthesis to 1012–1013 K, for hadronization, as shown in Table 2. Despite these differences, the structure of the RLM is quite similar to the structure of the Bohr model of the H atom as shown in Table 1. Only the equations of motion are different, since the two forces are different and in the case of the RLM Special Relativity must also be taken account for.
Interestingly, as also shown in Table 2, we have already found that, although the ΔH values vary so significantly, the − ΔS values for all these processes are of the order of a few kb per atom or hadron formed, i.e. 10–100 J/mol⋅K, since, due to the high temperatures involved, the ideal gas assumption provides a good approximation [9].
Due to their exothermic nature, both chemical synthesis and baryogenesis are favored at lower temperatures and thus a catalyst is required for these reactions to occur at significant rates. The necessity for efficient catalysts when dealing with exothermic synthesis reactions is shown schematically in Fig. 2 where the equilibrium reaction conversion, x, is plotted vs the dimensionless temperature Θ, defined from Θ = TΔS/ΔΗ. The figure also shows how the use of catalysts, characterized by an activation energy, EA, enables the approach to the equilibrium conversion curve.
Equilibrium conversion vs dimensionless temperature Θ showing the necessity of catalysis in the synthesis reactions of Table 2 which are all exothermic
As shown in Table 2, all the synthesis reactions, both in Chemistry and in Nuclear Physics, are exothermic (ΔΗ < 0) reactions. Consequently, as Fig. 2 shows, their equilibrium conversion decreases with increasing temperature. This creates the necessity of finding appropriate catalytic materials and appropriate reactor designs.
2.2 Temperature Programmed Kinetic Studies
There is a large wealth of well organized experimental data regarding the cross sections of hadronization reactions, e.g. Figure 3,top [16], which shows the rate [expressed as cross section, σ, measured in mbarn(= 10−31 m2)] of formation of various composite particles, from a beam comprising positrons (e+) and electrons (e−). These products include the Z boson, which has been recently shown via the RLM to be a rotational e+e−ν3 triad [13]. The two straight lines on the figure, as well as the temperature scale, have been drawn in the present study and, to the best of our knowledge, have not been drawn or discussed before. The two straight lines correspond to kinetic control at low temperatures, and to thermodynamic control, due to the exothermicity of the hadronization reaction, at higher temperatures. Thus, this figure is very similar to that of a temperature programmed reaction (TPR) spectrum for exothermic reactions in catalytic chemical synthesis. An example of a TPR spectrum in chemical catalysis is shown in Fig. 3, bottom, from the synthesis of or from the decomposition of, formic acid in presence of D2 on a Ni(110) catalyst.
There is a very rich literature about the catalysis of chemical synthesis [1], but very limited literature about the thermodynamics of quark-gluon condensation (baryogenesis) [9] and practically no literature about the catalysis of baryogenesis. We have recently shown [16] that positrons and electrons are extremely efficient catalysts for baryogenesis [16, 17] and, in fact, the TPR spectrum of Fig. 3, top demonstrates this in a very pronounced way.
Careful examination of the Tables of the decay products of composite particles [9] shows that there are only five elementary particles in our Universe, i.e. electrons, positrons and the three types of neutrinos. It thus appears that nature has been very generous in providing both the raw material (i.e. neutrinos) and the catalyst (i.e. electrons and positrons) for creating hadrons and our known universe.
2.3 Interpretation of the Product Spectra Generated via Annihilation of e+e− Pairs
So far there has not been any rigorous explanation of the very rich plethora of products obtained in the e+e− annihilation spectra of Fig. 3, top. It is vaguely believed that the energy produced by the exothermic “neutralization” of the e+, e− pair is somehow transformed into the masses of the components of the wide product spectrum of Fig. 3, top. But several questions arise, such as why is the Z boson the main synthesis product. A relatively recent (2018) study via the RLM of the structure of the Z boson provides a direct answer. The Z boson is a rotating \(e^+ - e^- - \nu_3\) triplet [13]. It is obviously formed by the direct interactions of the e+e− pair with one of the omnipresent ambient neutrinos. Furthermore, the subsequent creation in these experiments of a plethora of hadronization products is due to the well established catalytic action of electrons and positrons for hadronization which is due to the rapid acceleration of ambient neutrinos to highly relativistic velocities where hadronization via direct interactions between ultrarelativistic neutrinos takes place leading, in conjunction with the de Broglie equation of quantum mechanics [18], to hadron formation [16, 17] and to the unification of gravitational and nuclear forces [19].
3 Electrons and Positrons as Electrical and Gravitational Catalysts
3.1 Kinetic Similarities and the Role of Catalysts in Chemical and Nuclear Synthesis
A key aspect of many important synthesis reactions in Chemistry, such as the synthesis of NH3 from N2 and H2 and in Physics, such as the synthesis of a proton or neutron from three ultrarelativistic ν3 type, neutrinos which are the heaviest neutrinos, is the necessity of strong bonding between the reactant, e.g. N2 and H2 and the catalyst. This bonding is overall electrical in nature in the case of Chemistry (strong dissociative adsorption of N2 and H2 on the alkali doped catalyst) or gravitational in nature in the case of Physics (strong attraction of the reactant neutrinos with the e± catalyst). In this case the neutrinos are trapped gravitationally by the electron/positron which has a mass typically 11 orders of magnitude larger than the mass of a neutrino at rest. When this happens these relativistic neutrinos form rotating triads around the central electron/positron thus leading to the product (e.g. proton) formation [19]. This is similar to the formation of a H atom via Coulombic trapping of a rotating electron by the central proton in the Bohr model of the H atom (Table 1). As this Table also shows, the de Broglie equation is an essential part both of the H atom Bohr model and of the RLM. It ensures that the angular momentum, γmovr, of the rotating particles is an integer multiple of the Planck constant, and thus satisfies Heisenberg’s uncertainty conditions, expressing the dual wave-particle nature of the rotating leptons. It is worth noting that if the positron remains at the center of the rotating neutrino triad, then a proton is formed, and if it escapes then a neutron is formed. In the latter case the initial state of the catalyst (positron) is automatically restored, so the catalyst remains “unchanged”.
One thus observes that the catalytic action is electrostatic (Coulombic) in the case of NH3 synthesis and gravitational in the case of proton or neutron synthesis. It is truly remarkable that this dual catalytic action is accomplished by the same elementary particle (positron or electron). Nature is to be truly admired for this level of economy!
3.2 The Dual Catalytic Role of Electrons and Positrons
Electrons and positrons happen fortuitously to be important catalysts both for chemical and for nuclear reactions. In chemical catalysis due to their strong electrical charge which plays a key role in lowering the activation energy of many catalytic reactions. In nuclear catalysis involving neutrino hadronization, their unique catalytic role is due to their huge (in relation to neutrinos) gravitational mass which enables them to accelerate neutrinos to ultrarelativistic speeds [20,21,22], thus dramatically enhancing their mass and gravitational attraction, therefore leading to hadronization.
4 Conclusions
It is remarkable and philosophically interesting how electrons and positrons fortuitously combine these two very different catalytic properties which enable them to play a dominant role in our Universe both for forming hadrons and for forming chemical and biological products. Nature has provided a very efficient catalytic solution.
References
G. Ertl, H. Knözinger, F. Schüth (Eds.) in Handbook of Heterogeneous Catalysis, 2nd Ed., Vol. 1, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim (2008).
L. Pauling, The Nature of the Chemical Bond (3rd ed., Oxford University Press 1960) p. 111–120.
Vayenas CG, Bebelis S, Pliangos C, Brosda S, Tsiplakides D (2001) Electrochemical Activation of Catalysis: Promotion, Electrochemical Promotion and Metal-Support Interactions. Kluwer/Plenum Press, New York
Madix RJ (1979) Surface reactivity: heterogeneous reactions on single crystal surfaces. Acc Chem Res 12:265
Vayenas CG, Farr RD (1980) Cogeneration of electric energy and nitric oxide. Science 208:593
Vayenas CG, Bebelis S, Ladas S (1990) The dependence of catalytic activity on catalyst work function. Nature 343:625
Jiang Y, Yentekakis IV, Vayenas CG (1994) Methane to ethylene with 85% yield in a gas-recycle electrocatalytic reactor-separator. Science 264:1563
Vernoux Ph, Lizzaraga L, Tsampas MN, Sapountzi FM, De Lucas-Consuegra A, Valverde J-L, Souentie S, Vayenas CG, Tsiplakides D, Balomenou S, Baranova EA (2013) Ionically conducting ceramics as active catalyst supports. Chem Rev 113:8192
Griffiths D (2008) Introduction to elementary particles, 2nd edn. Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim
Vayenas CG, Souentie SN-A (2012) Gravity, special relativity and the strong force: A Bohr-Einstein-de Broglie model for the formation of hadrons. Springer, NY
Vayenas CG, Souentie S, Fokas A (2014) A Bohr-type model of a composite particle using gravity as the attractive force. Physica A 405:360
Vayenas CG, Fokas AS, Grigoriou D (2016) On the structure, mass and thermodynamics of the W± bosons. Physica A 450:37
Fokas AS, Vayenas CG (2016) On the structure, mass and thermodynamics of the Zo bosons. Physica A 464:231
Fokas AS, Vayenas CG, Grigoriou D (2018) On the mass and thermodynamics of the Higgs boson. Physica A 492:737
Vayenas CG, Fokas AS, Grigoriou D (2017) Catalysis and autocatalysis of chemical synthesis and of hadronization. Appl Catal B 203:582
Vayenas CG (2018) Thermodynamics and Catalysis of the Generation of Mass. Proc Acad of Athens 93A:97
Workman RL et al. (Particle Data Group) (2022) The review of particle physics. Prog. Theor Exp Phys 2022:083C01
de Broglie L (1923) Waves and quanta. Nature 112:540
Vayenas CG, Grigoriou D, Tsousis D, Parisis K, Aifantis EC (2022) Computation of the deuteron mass and force unification via the Rotating Lepton Model. Axioms 11:657. https://doi.org/10.3390/axioms11110657
Einstein A (1905) Zür Elektrodynamik bewegter Körper. Ann. der Physik. 17:891–921; English translation On the Electrodynamics of Moving Bodies by G.B. Jeffery and W. Perrett (1923)
French AP (1968) Special relativity. W.W. Norton and Co., New York
Freund J (2008) Special relativity for beginners. World Scientific Publishing, Singapore
Benziger JB, Madix RJ (1979) The Decomposition of Formic acid on Ni (100). Surf Sci 79:394–412
Funding
Open access funding provided by HEAL-Link Greece. No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vayenas, C.G., Tsousis, D. The Catalytic Role of Electrons and Positrons in the Synthesis of Chemicals and of Hadrons. Top Catal 66, 1280–1284 (2023). https://doi.org/10.1007/s11244-023-01812-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-023-01812-9