Abstract
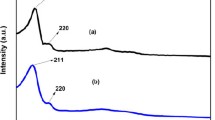
Surfactant addition during support synthesis resulted in materials with defined porosity, smaller particle size, and the absence of segregated ZrO2 and TiO2 crystalline phases at least to the XRD detection limit. The data obtained by N2 physisorption showed improved textural properties compared with the individual pure oxides. The Ultraviolet–visible (UV–Vis) diffuse spectroscopy revealed broadband assigned to the mixed-phase. Meanwhile, the micrographs obtained by transmission electron microscopy showed nanometric domains of crystallinity and a worm-like morphology in all the samples. Raman spectroscopy showed the possible formation of the TiZrO4 forming the mixed oxides support. The chemical analysis performed by ICP-OES corroborates the expected equimolar relation Zr/Ti and the atomic ratio Ni/(Ni + W) approximately equal to 0.31. The support properties intrinsically influenced the WS2 nano slabs, as observed by TEM micrographs for the NiW/ZT-x sulfided series. The XPS analysis revealed that sulfidation degree increases and the highest amount of NiWS phase among all the materials are observed when the NiW system is deposited on the mixed oxides prepared with pluronic. The latter was also related to the reaction rates, where the sulfided NiW/ZT-x catalysts presented higher values than the NiW/ZT reference material. NiW/ZT-P sulfided sample showed the maximum reaction rate among the series studied.











Similar content being viewed by others
References
Breysse M, Afanasiev P, Geantet C, Vrinat M (2003) Overview of support effects in hydrotreating catalysts. Catal Today 86:5–16. https://doi.org/10.1016/S0920-5861(03)00400-0
Breysse M, Geantet C, Afanasiev P, Blanchard J, Vrinat M (2008) Recent studies on the preparation, activation and design of active phases and supports of hydrotreating catalysts. Catal Today 130:3–13. https://doi.org/10.1016/J.CATTOD.2007.08.018
Barrera MC, Viniegra M, Escobar J, Vrinat M, de Reyes JAL, Murrieta F, García J (2004) Highly active MoS2 on wide-pore ZrO2-TiO2 mixed oxides. Catal Today 98:131–139. https://doi.org/10.1016/j.cattod.2004.07.027
Escobar J, De Reyes JAL, Ulín CA, Barrera MC (2013) Highly active sulfided CoMo catalysts supported on (ZrO2- TiO2)/Al2O3 ternary oxides. Mater Chem Phys 143:213–222. https://doi.org/10.1016/j.matchemphys.2013.08.054
Lu CM, Lin YM, Wang I (2000) Naphthalene hydrogenation over Pt/TiO2–ZrO2 and the behavior of strong metal—Support interaction (SMSI). Appl Catal A 198:223–234. https://doi.org/10.1016/S0926-860X(99)00515-3
Reddy BM, Khan A (2007) Science and recent advances on TiO2 -ZrO2 mixed oxides as catalysts and catalyst supports. Catal Rev 47:37–41. https://doi.org/10.1081/CR-200057488
Drisko GL, Luca V, Sizgek E, Scales N, Caruso RA (2009) Template synthesis and adsorption properties of hierarchically porous zirconium titanium oxides. Langmuir 25:5286–5293. https://doi.org/10.1021/la804030h
Díaz de León JN, Zavala-Sánchez LA, Suárez-Toriello VAA, Alonso-Núñez G, Zepeda TAA, Yocupicio RI, de Reyes JAL, Fuentes S (2017) Support effects of NiW catalysts for highly selective sulfur removal from light hydrocarbons. Appl Catal B Environ 213:167–176. https://doi.org/10.1016/j.apcatb.2017.05.014
de Soler-Illia GJAA, Sanchez C (2000) Interactions between poly(ethylene oxide)-based surfactants and transition metal alkoxides: their role in the templated construction of mesostructured hybrid organic-inorganic composites. New J Chem 24:493–499. https://doi.org/10.1039/b002518f
Díaz de León JN, Castañeda-García AL, Soto-Arteaga CE, Torres-Otañez G, Esqueda-Barrón Y, Guzmán-Cruz MA, Alonso-Nuñez G, Fuentes-Moyado S (2021) Selective removal of sulfur from 3-methyl thiophene under mild conditions over NiW/Al2O3-TiO2 modified by surfactants. Catal Today 377:59–68. https://doi.org/10.1016/j.cattod.2020.06.072
Rezaee S, Ranjbar K, Kiasat AR (2018) The effect of surfactant on the sol–gel synthesis of alumina-zirconia nanopowders. Ceram Int 44:19963–19969. https://doi.org/10.1016/j.ceramint.2018.07.263
Díaz de León JN, Zepeda TA, Alonso-Nuñez G, Galván DH, Pawelec B, Fuentes S (2015) Insight of 1D γ-Al2O3 nanorods decoration by NiWS nanoslabs in ultra-deep hydrodesulfurization catalyst. J Catal 321:51–61. https://doi.org/10.1016/j.jcat.2014.11.001
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Physica Status Solidi (b) 15:627–637. https://doi.org/10.1002/pssb.19660150224
Barton DG, Shtein M, Wilson RD, Soled SL, Iglesia E (1999) Structure and electronic properties of solid acids based on tungsten oxide nanostructures. J Phys Chem B 103:630–640. https://doi.org/10.1021/jp983555d
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Cychosz KA, Guillet-Nicolas R, García-Martínez J, Thommes M (2017) Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem Soc Rev 46:389–414. https://doi.org/10.1039/C6CS00391E
Sezgin Z, Yuksel N, Baykara T (2006) Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur J Pharm Biopharm 64:261–268. https://doi.org/10.1016/j.ejpb.2006.06.003
Díaz de León JN, Petranovskii V, De Reyes JAL, Alonso-Nuñez G, Zepeda TA, Fuentes S, García-Fierro JL (2014) One dimensional (1D) gamma-alumina nanorod linked networks: synthesis, characterization and application. Appl Catal A Gen 472:1–10. https://doi.org/10.1016/j.apcata.2013.12.005
Kosmulski M (2012) IEP as a parameter characterizing the pH-dependent surface charging of materials other than metal oxides. Adv Coll Interface Sci 171–172:77–86. https://doi.org/10.1016/j.cis.2012.01.005
Regazzoni AE, Blesa MA, Maroto AJG (1983) Interfacial properties of zirconium dioxide and magnetite in water. J Colloid Interface Sci 91:560–570. https://doi.org/10.1016/0021-9797(83)90370-3
Maity SK, Rana MS, Bej SK, Ancheyta-Juárez J, Dhar GM, Rao TSRP (2001) TiO2–ZrO2 mixed oxide as a support for hydrotreating catalyst. Catal Lett 72:115–119. https://doi.org/10.1023/A:1009045412926
Geobaldo F, Bordiga S, Zecchina A, Giamello E, Leofanti G, Petrini G (1992) DRS UV-Vis and EPR spectroscopy of hydroperoxo and superoxo complexes in titanium silicalite. Catal Lett 16:109–115. https://doi.org/10.1007/BF00764360
López EF, Escribano VS, Panizza M, Carnasciali MM, Busca G (2001) Vibrational and electronic spectroscopic properties of zirconia powders. J Mater Chem 11:1891–1897. https://doi.org/10.1039/b100909p
Ilie AG, Scarisoareanu M, Morjan I, Dutu E, Badiceanu M, Mihailescu I (2017) Principal component analysis of Raman spectra for TiO2 nanoparticle characterization. Appl Surf Sci 417:93–103. https://doi.org/10.1016/j.apsusc.2017.01.193
Ding S, Zhao J, Yu Q (2019) Effect of zirconia polymorph on vapor-phase ketonization of propionic acid. Catalysts 9:17–22. https://doi.org/10.3390/catal9090768
Mokrushin AS, Simonenko EP, Simonenko NP, Bukunov KA, Gorobtsov PY, Sevastyanov VG, Kuznetsov NT (2019) Gas-sensing properties of nanostructured TiO 2-xZrO 2 thin films obtained by the sol-gel method. J Sol-Gel Sci Technol 92:415–426. https://doi.org/10.1007/s10971-019-04979-4
Díaz de León JN, Picquart M, Villarroel M, Vrinat M, Llambias FJG, Murrieta F, de Reyes JAL (2010) Effect of gallium as an additive in hydrodesulfurization WS2/γ-Al2O3 catalysts. J Mol Catal A Chem 323:1–6. https://doi.org/10.1016/J.MOLCATA.2010.03.008
Gervasini A, Auroux A (1991) Acidity and basicity of metal oxide surfaces, II. Determination Catal Decomposition Isopropanol 198:190–198
Díaz de León JN, Antunes-García J, Alonso-Nuñez G, Zepeda TA, Galvan DH, de Reyes JAL, Fuentes S (2018) Support effects of NiW hydrodesulfurization catalysts from experiments and DFT calculations. Appl Catal B Environ 238:480–490. https://doi.org/10.1016/j.apcatb.2018.07.059
Vrinat M, Breysse M, Geantet C, Ramirez J, Massoth F (1994) Effect of MoS2 morphology on the HDS activity of hydrotreating catalysts. Catal Lett 26:25–35. https://doi.org/10.1007/BF00824029
Rodríguez-Castellón E, Jiménez-López A, Eliche-Quesada D (2008) Nickel and cobalt promoted tungsten and molybdenum sulfide mesoporous catalysts for hydrodesulfurization. Fuel 87:1195–1206. https://doi.org/10.1016/J.FUEL.2007.07.020
Coulier L, Kishan G, van Veen JAR, Niemantsverdriet JW (2002) Influence of support-interaction on the sulfidation behavior and hydrodesulfurization activity of Al2O3-supported W, CoW, and NiW model catalysts. J Phys Chem B 106:5897–5906. https://doi.org/10.1021/jp0136821
Reinhoudt HR, Crezee E, van Langeveld AD, Kooyman PJ, van Veen JAR, Moulijn JA (2000) Characterization of the Active Phase in NiW/γ-Al2O3 Catalysts in Various Stages of Sulfidation with FTIR(NO) and XPS. J Catal 196:315–329. https://doi.org/10.1006/JCAT.2000.3042
Fairley N, Fernandez V, Richard-Plouet M, Guillot-Deudon C, Walton J, Smith E, Flahaut D, Greiner M, Biesinger M, Tougaard S, Morgan D, Baltrusaitis J (2021) Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl Surface Sci Adv. https://doi.org/10.1016/j.apsadv.2021.100112
Díaz de León JN, Picquart M, Massin L, Vrinat M, De Reyes JAL (2012) Hydrodesulfurization of sulfur refractory compounds: Effect of gallium as an additive in NiWS/gamma-Al2O3 catalysts. J Mol Catal A Chem. https://doi.org/10.1016/j.molcata.2012.07.006
Gionco C, Battiato A, Vittone E, Paganini MC, Giamello E (2013) Structural and spectroscopic properties of high temperature prepared ZrO2-TiO2 mixed oxides. J Solid State Chem 201:222–228. https://doi.org/10.1016/j.jssc.2013.02.040
Barrera MC, Escobar J, de Reyes JAL, Cortés MA, Viniegra M, Hernández A (2006) Effect of solvo-thermal treatment temperature on the properties of sol-gel ZrO2-TiO2 mixed oxides as HDS catalyst supports. Catal Today 116:498–504. https://doi.org/10.1016/j.cattod.2006.06.030
Acknowledgements
We thank D. Dominguez, J. A. Díaz, E. Flores, F. Ruiz, I. Gradilla, J. Peralta, and E. Aparicio for their expert technical assistance. CESA expresses his gratitude to CONACyT for the Ph. D scholarship with number 929101. YEB acknowledges the Postdoctoral fellowship from DGAPA-UNAM.
Funding
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) 117373, and Direccion General de Asuntos del Personal Academico (DGPA)-Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) IN-104122 projects.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soto-Arteaga, C.E., Guzmán-Cruz, M.A., Esqueda-Barron, Y. et al. Triblock Copolymer Effect During the Synthesis of ZrO2-TiO2 Mixed Oxides Supports for NiW Hydrodesulfurization Catalysts. Top Catal 65, 1516–1529 (2022). https://doi.org/10.1007/s11244-022-01649-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01649-8