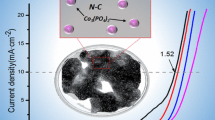
Abstract
In order to obtain platinum-group-metal-free catalysts for the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER) in alkaline electrolyzers, nitrogen-doped mesoporous carbons were prepared from pyrolysis of two cobalt metal organic frameworks (MOFs), one linear coordination polymer and one complex. The catalyst derived from cobalt 2,3-pyrazinedicarboxylate polymer (700 °C) had a Tafel slope of 90 mV dec−1 and 130 mV dec−1 for the HER and OER respectively, a current density of 10 mA cm−2 was reached at − 0.23 V and 1.56 V vs RHE for the HER and OER respectively. In order to prove the bifunctional catalytic activity towards the overall water splitting, a two electrode electrolyzer was constructed depositing the catalyst on carbon paper. H2 and O2 evolved volumes followed the Faraday law, showing efficiency very close to 100%. The same materials also showed catalytic activity towards the oxygen reduction reaction (ORR), reaching an electron transfer number close to 4 and low H2O2 yields. The acid leached catalyst derived from Cobalt 2,3-pyrazinedicarboxylate polymer (700 °C) reached a ∆E = Ej = 10(OER) −E½(ORR) = 0.80 V, making it an useful oxygen catalyst for metal-air batteries.




reproduced with permission from ref [31]. Copyright 2017 Elsevier. b Linear sweep voltammograms on the Co(CO2)2Pz 700 °C (not acid leached) derived catalysts at different rotation speeds (ω). c Koutecky-Levich plot showing the linear relation between j−1 and ω−1/2 for the ORR on the different studied catalysts at 0.6 V vs RHE. Electron transfer numbers are shown on the plot. d Percentage of H2O2 produced as function of the disk potential at 100 rpm disk rotation rate for the ORR on the studied catalysts



Similar content being viewed by others
References
Schlögl R (2022) Chemical energy storage. De Gruyter, Berlin
Smolinka T, Garche J (2022) Electrochemical power sources: fundamentals, systems, and applications hydrogen production by water electrolysis. Elsevier, Amsterdam
Napporn TW, Holade Y (2021) Metal oxide-based nanostructured electrocatalysts for fuel cells, electrolyzers, and metal-air batteries. Elservier, Amsterdam
Santhanam KSV, Press RJ, Miri MJ, Bailey AV, Takacs GA (2017) Introduction to hydrogen technology. Wiley, New York
Chen D, Chen C, Baiyee ZM, Shao Z, Ciucci F (2015) Nonstoichiometric oxides as low-cost and highly-efficient oxygen reduction/evolution catalysts for low-temperature electrochemical devices. Chem Rev 115:9869–9921. https://doi.org/10.1021/acs.chemrev.5b00073
Zhang J, Chen G, Müllen K, Feng X (2018) Carbon-rich nanomaterials: fascinating hydrogen and oxygen electrocatalysts. Adv Mater 30:1800528. https://doi.org/10.1002/adma.201800528
Meng Y, Huang X, Lin H, Zhang P, Gao Q, Li W (2019) Carbon-based nanomaterials as sustainable noble-metal-free electrocatalysts. Front Chem 7:759
Batten SR, Champness NR, Chen X-M, Garcia-Martinez J, Kitagawa S, Öhrström L, O’Keeffe M, Paik Suh M, Reedijk J (2013) Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl Chem 85:1715–1724
Kaskel S (2016) The chemistry of metal-organic frameworks: synthesis, characterization, and applications. Wiley-VCH, Weinheim
Fan W, Zhang X, Kang Z, Liu X, Sun D (2021) Isoreticular chemistry within metal–organic frameworks for gas storage and separation. Coord Chem Rev 443:213968. https://doi.org/10.1016/j.ccr.2021.213968
Rojas S, Arenas-Vivo A, Horcajada P (2019) Metal-organic frameworks: a novel platform for combined advanced therapies. Coord Chem Rev 388:202–226. https://doi.org/10.1016/j.ccr.2019.02.032
Stassen I, Burtch N, Talin A, Falcaro P, Allendorf M, Ameloot R (2017) An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem Soc Rev 46:3185–3241. https://doi.org/10.1039/C7CS00122C
Fang R, Dhakshinamoorthy A, Li Y, Garcia H (2020) Metal organic frameworks for biomass conversion. Chem Soc Rev 49:3638–3687. https://doi.org/10.1039/D0CS00070A
Liu H, Wang Y, Qin Z, Liu D, Xu H, Dong H, Hu W (2021) Electrically conductive coordination polymers for electronic and optoelectronic device applications. J Phys Chem Lett 12:1612–1630. https://doi.org/10.1021/acs.jpclett.0c02988
Duan J, Chen S, Zhao C (2017) Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat Commun 8:15341. https://doi.org/10.1038/ncomms15341
Wei Y, Ren X, Ma H, Sun X, Zhang Y, Kuang X, Yan T, Wu D, Wei Q (2018) In situ Formed Co(TCNQ)2 metal-organic framework array as a high-efficiency catalyst for oxygen evolution reactions. Chemistry 24:2075–2079. https://doi.org/10.1002/chem.201705606
Huang J, Li Y, Huang R-K, He C-T, Gong L, Hu Q, Wang L, Xu Y-T, Tian X-Y, Liu S-Y, Ye Z-M, Wang F, Zhou D-D, Zhang W-X, Zhang J-P (2018) Electrochemical exfoliation of pillared-layer metal-organic framework to boost the oxygen evolution reaction. Angew Chem Int Ed 57:4632–4636. https://doi.org/10.1002/anie.201801029
Guan BY, Yu XY, Bin WuH, Lou XW (2017) Complex nanostructures from materials based on metal-organic frameworks for electrochemical energy storage and conversion. Adv Mater 29:1703614. https://doi.org/10.1002/adma.201703614
Li X, Niu Z, Jiang J, Ai L (2016) Cobalt nanoparticles embedded in porous N-rich carbon as an efficient bifunctional electrocatalyst for water splitting. J Mater Chem A 4:3204–3209. https://doi.org/10.1039/C6TA00223D
Jiao L, Zhou Y-X, Jiang H-L (2016) Metal–organic framework-based CoP/reduced graphene oxide: high-performance bifunctional electrocatalyst for overall water splitting. Chem Sci 7:1690–1695. https://doi.org/10.1039/C5SC04425A
Guan C, Xiao W, Wu H, Liu X, Zang W, Zhang H, Ding J, Feng YP, Pennycook SJ, Wang J (2018) Hollow Mo-doped CoP nanoarrays for efficient overall water splitting. Nano Energy 48:73–80. https://doi.org/10.1016/j.nanoen.2018.03.034
Yu Z, Bai Y, Zhang S, Liu Y, Zhang N, Wang G, Wei J, Wu Q, Sun K (2018) Metal-organic framework-derived Co3ZnC/Co embedded in nitrogen-doped carbon nanotube-grafted carbon polyhedra as a high-performance electrocatalyst for water splitting. ACS Appl Mater Interfaces 10:6245–6252. https://doi.org/10.1021/acsami.7b16130
Feng X, Bo X, Guo L (2018) CoM(M=Fe, Cu, Ni)-embedded nitrogen-enriched porous carbon framework for efficient oxygen and hydrogen evolution reactions. J Power Sources 389:249–259. https://doi.org/10.1016/j.jpowsour.2018.04.027
Aijaz A, Masa J, Rösler C, Xia W, Weide P, Botz AJR, Fischer RA, Schuhmann W, Muhler M (2016) Co@Co3O4 encapsulated in carbon nanotube-grafted nitrogen-doped carbon polyhedra as an advanced bifunctional oxygen electrode. Angew Chem Int Ed 55:4087–4091. https://doi.org/10.1002/anie.201509382
Cao Z, Zhou T, Xi W, Zhao Y (2018) Bimetal metal-organic frameworks derived Co0.4Fe0.28P and Co0.37Fe0.26S nanocubes for enhanced oxygen evolution reaction. Electrochim Acta 263:576–584. https://doi.org/10.1016/j.electacta.2017.11.058
Hao Y, Xu Y, Liu W, Sun X (2018) Co/CoP embedded in a hairy nitrogen-doped carbon polyhedron as an advanced tri-functional electrocatalyst. Mater Horizons 5:108–115. https://doi.org/10.1039/C7MH00706J
Yang M, Zhang Y, Jian J, Fang L, Li J, Fang Z, Yuan Z, Dai L, Chen X, Yu D (2019) Donor-acceptor nanocarbon ensembles to boost metal-free all-pH hydrogen evolution catalysis by combined surface and dual electronic modulation. Angew Chem Int Ed 58:16217–16222. https://doi.org/10.1002/anie.201907826
Lu X, Yim W-L, Suryanto BHR, Zhao C (2015) Electrocatalytic oxygen evolution at surface-oxidized multiwall carbon nanotubes. J Am Chem Soc 137:2901–2907. https://doi.org/10.1021/ja509879r
Zheng Y, Jiao Y, Zhu Y, Li LH, Han Y, Chen Y, Du A, Jaroniec M, Qiao SZ (2014) Hydrogen evolution by a metal-free electrocatalyst. Nat Commun 5:3783. https://doi.org/10.1038/ncomms4783
Díaz-Duran AK, Montiel G, Viva FA, Roncaroli F (2019) Co, N-doped mesoporous carbons cobalt derived from coordination polymer as supercapacitors. Electrochim Acta 299:987–998. https://doi.org/10.1016/j.electacta.2019.01.023
Díaz-Duran AK, Roncaroli F (2017) MOF derived mesoporous nitrogen doped carbons with high activity towards oxygen reduction. Electrochim Acta 251:638–650. https://doi.org/10.1016/j.electacta.2017.08.055
Díaz-Duran AK, Viva FA, Roncaroli F (2019) High durability fuel cell cathodes obtained from cobalt metal organic frameworks. Electrochim Acta 320:134623. https://doi.org/10.1016/j.electacta.2019.134623
Rahul B, Anh P, Bo W, Carolyn K, Hiroyasu F, Michael O, Yo M (2008) High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319:939–943. https://doi.org/10.1126/science.1152516
Wang X, Zhou J, Fu H, Li W, Fan X, Xin G, Zheng J, Li X (2014) MOF derived catalysts for electrochemical oxygen reduction. J Mater Chem A 2:14064–14070. https://doi.org/10.1039/C4TA01506A
Xia W, Zhu J, Guo W, An L, Xia D, Zou R (2014) Well-defined carbon polyhedrons prepared from nano metal–organic frameworks for oxygen reduction. J Mater Chem A 2:11606–11613. https://doi.org/10.1039/C4TA01656D
Liu Y-H, Tsai H-L, Lu Y-L, Wen Y-S, Wang J-C, Lu K-L (2001) Assembly of a robust, thermally stable porous Cobalt(II) nicotinate framework based on a dicobalt carboxylate unit. Inorg Chem 40:6426–6431. https://doi.org/10.1021/ic010528k
O’Connor CJ, Sinn E (1981) Crystal structures and magnetic properties of cobalt(II) pyrazinecarboxylate and pyrazinedicarboxylate complexes. Inorg Chem 20:545–551. https://doi.org/10.1021/ic50216a046
Mao L, Rettig SJ, Thompson RC, Trotter J, Xia S (1996) 2,3-Pyrazinedicarboxylates of cobalt(II), nickel(II), and copper(II); magnetic properties and crystal structures. Can J Chem 74:433–444. https://doi.org/10.1139/v96-047
Franceschini EA, Lacconi GI, Corti HR (2015) Kinetics of the hydrogen evolution on nickel in alkaline solution: new insight from rotating disk electrode and impedance spectroscopy analysis. Electrochim Acta 159:210–218. https://doi.org/10.1016/j.electacta.2015.01.110
Vos JG, Koper MTM (2019) Examination and prevention of ring collection failure during gas-evolving reactions on a rotating ring-disk electrode. J Electroanal Chem 850:113363. https://doi.org/10.1016/j.jelechem.2019.113363
Wei J, Zhou M, Long A, Xue Y, Liao H, Wei C, Xu ZJ (2018) Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions. Nano-Micro Lett 10:75. https://doi.org/10.1007/s40820-018-0229-x
Xu M, Han L, Han Y, Yu Y, Zhai J, Dong S (2015) Porous CoP concave polyhedron electrocatalysts synthesized from metal–organic frameworks with enhanced electrochemical properties for hydrogen evolution. J Mater Chem A 3:21471–21477. https://doi.org/10.1039/C5TA05018A
Gennero de Chialvo MR, Chialvo AC (2018) Advances in the kinetic study of the hydrogen electrode reaction on metals: theoretical and experimental aspects. In: Wandelt K (ed) Encyclopedia of interfacial chemistry: surface science and electrochemistry. Elsevier, Amsterdam, pp 345–360
You B, Jiang N, Sheng M, Gul S, Yano J, Sun Y (2015) High-performance overall water splitting electrocatalysts derived from cobalt-based metal-organic frameworks. Chem Mater 27:7636–7642. https://doi.org/10.1021/acs.chemmater.5b02877
Feng C, Xie Y, Qiao S, Guo Y, Li S, Zhang L, Wang W, Wang J (2021) Porous MoWN/MoWC@NC Nano-octahedrons synthesized via confined carburization and vapor deposition in MOFs as efficient trifunctional electrocatalysts for oxygen reversible catalysis and hydrogen production in the same electrolyte. J Colloid Interface Sci 601:626–639. https://doi.org/10.1016/j.jcis.2021.05.133
Li S, Wang X, Li M, Liu J, Li C, Wu H, Guo D, Ye F, Wang S, Cheng L, Liu A (2019) Self-supported ternary (NixFey)2P nanoplates arrays as an efficient bifunctional electrocatalyst for overall water splitting. Electrochim Acta 319:561–568. https://doi.org/10.1016/j.electacta.2019.07.022
Lu X, Zhao C (2015) Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat Commun 6:6616. https://doi.org/10.1038/ncomms7616
Yan Z, Liu H, Hao Z, Yu M, Chen X, Chen J (2020) Electrodeposition of (hydro)oxides for an oxygen evolution electrode. Chem Sci 11:10614–10625. https://doi.org/10.1039/D0SC01532F
Meng Z, Chen N, Cai S, Wang R, Wu J, Tang H (2021) Recent advances of hierarchically porous bifunctional oxygen electrocatalysts derived from metal–organic frameworks for Zn–air batteries. Mater Chem Front 5:2649–2667. https://doi.org/10.1039/D0QM00878H
Song Y, Yang Y, Mo S, Guo D, Liu L (2022) Fast construction of (Fe2O3)x@Ni-MOF heterostructure nanosheets as highly active catalyst for water oxidation. J Alloys Compd 892:162149. https://doi.org/10.1016/j.jallcom.2021.162149
Qi Q, Hu J, Guo S, Song H, Wang S, Yao Y, Le T, Li W, Zhang C, Zhang L (2021) Large-scale synthesis of low-cost bimetallic polyphthalocyanine for highly stable water oxidation. Appl Catal B 299:120637. https://doi.org/10.1016/j.apcatb.2021.120637
Suen N-T, Hung S-F, Quan Q, Zhang N, Xu Y-J, Chen HM (2017) Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem Soc Rev 46:337–365. https://doi.org/10.1039/C6CS00328A
Wang Y, Jin W, Xuan C, Wang J, Li J, Yu Q, Li B, Wang C, Cai W, Wang J (2021) In-situ growth of CoFeS2 on metal-organic frameworks-derived Co-NC polyhedron enables high-performance oxygen electrocatalysis for rechargeable zinc-air batteries. J Power Sources 512:230430. https://doi.org/10.1016/j.jpowsour.2021.230430
Lei Z, Jin X, Li J, Liu Y, Liu J, Jiao S, Cao R (2022) Tuning electrochemical transformation process of zeolitic imidazolate framework for efficient water oxidation activity. J Energy Chem 65:505–513. https://doi.org/10.1016/j.jechem.2021.06.019
Wang B, Han X, Guo C, Jing J, Yang C, Li Y, Han A, Wang D, Liu J (2021) Structure inheritance strategy from MOF to edge-enriched NiFe-LDH array for enhanced oxygen evolution reaction. Appl Catal B 298:120580. https://doi.org/10.1016/j.apcatb.2021.120580
Wang H, Chen B-H, Liu D-J (2021) Metal-organic frameworks and metal-organic gels for oxygen electrocatalysis: structural and compositional considerations. Adv Mater 33:2008023. https://doi.org/10.1002/adma.202008023
Arce MD, Fernández JL (2015) Oxygen reduction to water operating through the Direct (or Dissociative) Route: descriptive and fitting capabilities of polarization curves. Electrochim Acta 182:953–962. https://doi.org/10.1016/j.electacta.2015.09.167
Jaouen F, Dodelet J-P (2009) O2 reduction mechanism on non-noble metal catalysts for PEM fuel cells. Part I: experimental rates of O2 electroreduction, H2O2 electroreduction, and H2O2 disproportionation. J Phys Chem C 113:15422–15432. https://doi.org/10.1021/jp900837e
Zhu Y, Yue K, Xia C, Zaman S, Yang H, Wang X, Yan Y, Xia BY (2021) Recent advances on MOF derivatives for non-noble metal oxygen electrocatalysts in zinc-air batteries. Nano-Micro Lett 13:137. https://doi.org/10.1007/s40820-021-00669-5
Choi HC, Jung YM, Noda I, Bin KS (2003) A study of the mechanism of the electrochemical reaction of lithium with CoO by two-dimensional soft X-ray Absorption Spectroscopy (2D XAS), 2D Raman, and 2D Heterospectral XAS-raman correlation analysis. J Phys Chem B 107:5806–5811. https://doi.org/10.1021/jp030438w
Hadjiev VG, Iliev MN, Vergilov IV (1988) The Raman spectra of Co3O4. J Phys C 21:L199–L201. https://doi.org/10.1088/0022-3719/21/7/007
Liu Y-C, Koza JA, Switzer JA (2014) Conversion of electrodeposited Co(OH)2 to CoOOH and Co3O4, and comparison of their catalytic activity for the oxygen evolution reaction. Electrochim Acta 140:359–365. https://doi.org/10.1016/j.electacta.2014.04.036
Franceschini EA, Bruno MM, Viva FA, Williams FJ, Jobbágy M, Corti HR (2012) Mesoporous Pt electrocatalyst for methanol tolerant cathodes of DMFC. Electrochim Acta 71:173–180. https://doi.org/10.1016/j.electacta.2012.03.121
Acknowledgements
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (Project PICT-2016-3017), the Commission for Atomic Energy of Argentina (CNEA), and the Research Council of Argentina (CONICET). FR is a researcher of CONICET and A.K.D.D. held a doctoral fellowship of this institution. The authors acknowledge Daniel R. Vega for obtaining the XRD patterns, and Silvia A. Dominguez, for the SEM images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Díaz-Duran, A.K., Iadarola-Pérez, G., Halac, E.B. et al. Trifunctional Catalysts for Overall Water Splitting and Oxygen Reduction Reaction Derived from Co,Ni MOFs. Top Catal 65, 887–901 (2022). https://doi.org/10.1007/s11244-022-01611-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01611-8