Abstract
This paper presents a brief review of some research projects carried out in the author’s laboratories over a number of years. The work reported concerns the use of nickel-containing catalysts for a range of C1 reactions: the steam reforming of methane, the methanation of CO, the oxidative coupling of methane and the dry reforming of methane. A number of novel catalysts have been developed in the course of this work, mostly in collaborative projects with industrial organisations, and some of the background to this work is discussed. The paper emphasises the importance in work of the type described of the establishment of contacts between the academic laboratory and industrial researchers such as Mike Spencer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
This contribution is a short personal review discussing some aspects of the research work focussed on the preparation and uses of nickel-containing catalysts for C1 reactions that has been carried out by the author’s various research groups during his career at the Universities of Bradford, Twente and Limerick. The review covers processes involving either the reaction or production of methane: the steam reforming of methane, the methanation of CO, methane coupling to produce C2 molecules, and the CO2 (dry) reforming of methane. The importance of maintaining industrial contacts at all stages of any such work and thus gaining a better understanding of the needs of industry is stressed. Not only do such contacts with industrial laboratories ensure the relevance of any work but they contribute to the more general development of the researchers involved, both students and postdoctoral assistants. Many of those who contributed to the work described in this review have progressed to senior positions in industrial organisations, encouraged and helped by such contacts.
2 Coprecipitated Nickel-Alumina Catalysts for Steam Reforming and Related Reactions
This author’s first real encounter with catalysis (as opposed to his previous work adsorption on clean metal surfaces) came in a project on the steam reforming of methane (Eq. 1), an endothermic reaction that is thermodynamically reversible at lower temperatures:
The initial work focussed on the use of a coprecipitated potassium-doped Ni-alumina material, the so-called Catalytic Rich Gas (CRG) catalyst [1]. This had been developed by British Gas for the steam reforming of naphtha to form methane-rich gases and was supplied in the form of pellets, examples of which are shown on the left-hand side of Fig. 1. The catalyst contains a high proportion of Ni (75% by weight in the reduced form) and the potassium is added to the material after precipitation and washing of the precipitate but before calcination. It has the function of reducing carbon deposition on the catalyst under operation conditions. (Ridler and Twigg have given a useful description to the industrial background to the CRG catalyst [2].) This catalyst was being produced for commercial use by Laporte Industries of Widnes, Lancashire. Together with Mike Steel, the first research student to work on this topic in Bradford, a project was carried out in cooperation with them to examine the possible use of the CRG catalyst for the steam reforming of methane to produce syngas (a mixture of hydrogen and carbon monoxide). We also compared the behaviours of the CRG catalyst catalyst with those of more conventional impregnated nickel-alumina materials. In order to carry out this work, we built a glass high vacuum system that allowed the reaction to be studied at relatively low total pressures (ca. 0.01 atm) and at temperatures up to about 600 °C. (These are much lower pressures and temperatures than used industrially for the steam reforming process but the use of such a low pressure allowed the reaction to be carried out under essentially irreversible conditions and under effectively kinetically controlled rather than thermodynamically controlled conditions.) Gas analysis was carried out using a small mass spectrometer originally designed for residual pressure measurements and the system included a simple glass recirculating pump to ensure adequate gas mixing. It was found that it was important to carry out the kinetic measurements under fully reducing conditions using a carefully reduced catalyst sample (particle size 0.25–0.35 mm); this necessitated the addition of small quantities of hydrogen to the reaction mixture (added in each experiment immediately following initial reduction of the catalyst in hydrogen (ca. 0.03 atm) at 600 °C).
Samples of catalysts supplied by Laporte Industries. From left: pellets of the commercial CRG catalyst; the uncalcined precipitate from which the catalyst pellets were produced; extrudates of a commercial hydrodesulphurisation catalyst used for the removal of sulphur from hydrocarbon feeds, this also produced by Laporte Industries
Experiments carried out with additions of D2 or D2O to the reaction mixture showed that there was no exchange into the methane but that the water and the product hydrogen were completely equilibrated. The results were therefore consistent with the rate determining step under our conditions being the adsorption of methane on the reduced Ni surface of the catalyst. Although detailed kinetic measurements were made, in retrospect it is recognised that the results may have been to some extent affected by internal diffusion limitations as the measured activation energy for the reaction was 29 kJ mol−1, much lower than the value of 130 kJ mol−1 reported for the reaction over a Ni foil [3].
Detailed kinetic measurements were also carried out for comparison purposes with a series of impregnated catalyst samples prepared using a variety of different aluminas as supports [4]. It was found that the orders of reaction and activation energies for these materials were very different from those with the CRG catalyst and it was suggested that the active sites on the fully reduced impregnated samples prepared with all the different supports involved highly dispersed Ni atoms, probably derived by the reduction of surface nickel aluminate species as shown in Fig. 2. That oxygen species associated with such sites participated in the reaction on these catalysts was shown by exchange experiments in which 16O from the fully reduced catalyst was found among the products of the steam reforming reaction carried out with H218O. The differences between the CRG catalyst and the impregnated materials were most unlikely to have been caused by the possible diffusion limitations noted above. These results and some aspects of the scientific literature on the steam reforming of hydrocarbons were reported in a review article published in 1975 [5].
At that time, the author’s contacts in Laporte informed him that they had encountered a problem with the production of the CRG catalyst which at that time was used for the production of synthetic natural gas (SNG) from naphtha feedstocks. The operator of the precipitation plant had encountered difficulties in filtering the coprecipitate after the preparation step and so had changed the precipitation conditions to improve the rate of filtration. However, the resultant catalyst had been found to give poorer conversions and yields in the production of methane-rich gases than the one that had been produced previously. We therefore started further collaborative work making use of existing conventional thermal analysis methods in a way that permitted samples to be characterised and tested for CO methanation very quickly, this being an early precursor of high-throughput experimentation; we were able to examine daily a significant number of samples from a large range of materials prepared for us by our colleagues in Laporte [6]. In essence, these experiments involved: (a) the use of a differential scanning calorimeter (a Perkin Elmer DSC 1b which contained two sample pans housed in an enclosure through which gases could be flowed) to examine the decomposition of the precipitates; (b) reduction of the samples in a separate system and passivation in a nitrogen flow containing very low oxygen concentrations; (c) re-reduction of the sample in the DSC system at a temperature of 500 °C, this being followed by examination of the temperature dependence of the exotherm due to the methanation of CO in the same system. The highly exothermic methanation reaction (the reverse of the steam reforming of methane, a reversible reaction if carried out at higher temperatures):
was carried out using a feed supplied from a pre-mixed cylinder of hydrogen and CO (3:1) supplied by BOC without any added carrier gas and the rate of evolution of heat in the reaction, proportional to the rate of reaction, was measured as a function of temperature. The advantage of the DSC 1b in studying this reaction was that both the catalyst and a reference sample of alumina were exposed simultaneously to the same reaction mixture in the sample cell. Some typical results are shown very schematically in Fig. 3. Figure 3a represents the type of data obtained during the endothermic decomposition of samples of three different materials in the DSC: Al(OH)3, a basic nickel carbonate, and a coprecipitated Ni/Al material. The first endothermic peak found for the coprecipitate at around 100 °C corresponds to loss of water of crystallisation while the peaks at the higher temperatures correspond to the decomposition of hydroxide species. It is clear that the decomposition pattern of the Ni/Al material is significantly different from those of its component species and we took this to indicate that a compound of some kind, which we at that time called a “nickel hydro aluminate”, had been formed. (A German patent that appeared at about that time [7] showed that this material had a well-defined mineral structure, that of “tacovite”, Ni6Al2(OH)16·CO3·4H2O; the importance of this structure will be discussed further below.) Figure 3b represents some DSC results for a reduced Ni/Al sample showing that methanation commenced with this sample at about 200 °C. Results of this type turned out to be highly reproducible and we were able, by comparing the light-off temperatures and also the kinetic behaviour over the lower range of reaction temperatures (from which activation energy plots could be constructed), to examine topics such as the effect of carbiding the sample by stopping the flow and exposing it to the methane formed to examine whether or not carbon deposition would cause any significant changes to the catalyst. As shown in Fig. 3b, the activity of the catalyst was reduced significantly when carbided but the original activity could be at least partially restored by exposure once more to the CO/H2 reaction mixture. It is therefore unlikely that any significant carbiding occurred during the methanation reaction. This rapid screening work allowed us to show that the reduced activity of the modified production catalyst was due to a change in the pH used in the precipitation procedure, this probably resulting in retention of nitrate anions in the hydroaluminate structure of the precipitate. Our industrial collaborators worked very closely with us at all stages of this work. Careful control of the precipitation conditions and the subsequent calcination step to avoid the retention of nitrate anions led to catalysts with consistently better activities.
Further development of the rapid screening methodology, now using a combination of thermogravimetric analysis (TGA) to follow the reduction behaviour and the DSC to examine the methanation reaction for each of the reduced samples, was then applied in the next phase of our work on coprecipitated Ni-alumina catalysts. This was part of a collaborative project funded by the NATO Advanced Scientific Research Programme and involving Lou van Reijen and Giel Doesburg in T.U. Delft (NL) and Bernd Höhlein and Reinhard Menzer of the KFA, Juelich (Germany). The work took advantage of our experience using methanation as a test reaction and targeted a greater understanding of the Ni-alumina catalyst system, the intention being to apply such a catalyst in the Adam and Eve concept shown in Fig. 4 [8]. (ADAM stands for Adiabatic Methanation reactor; EVA (in English EVE) stands for Einzelrohr–Versuchs–Anlage.) In this system, the steam reforming reactor was to be heated using He from a high-temperature nuclear reactor and the resultant CO + H2 mixture was to be transported to a methanation system where the reverse reaction was to be carried out. In this way, the energy from the nuclear reactor could be transported to a site remote from the nuclear reactor. (The Adam and Eve system was later progressed to a pilot plant stage using catalysts from Haldor Topsoe in the methanation reactors. It was operated very successfully using electrical energy to heat the steam reformer (EVA), electricity being generated once more with the energy produced in the methanation system (ADAM)). The conditions in the ADAM reactor are very different to those encountered in the methanation systems used for removing traces of CO from the feed to an ammonia plant: the temperature rise in the first reactor of the Adam can be very high and hence gas circulation is introduced to ensure that an entrance temperature of about 350 °C is maintained together with an exit temperature about of 600 °C. The requirement of any methanation catalyst was that it would operate with a 3:1 H2/CO feed without diluent and have activity at 350 °C while having long-term stability at 600 °C. (In practice, the feed reaching the methanation reactor in such a system includes traces of unreacted methane and water as well as some CO2 [9]. As these molecules are formed as products of the methanation reaction (assuming that CO2 is also formed by the water-gas shift reaction), their effects were not examined in our work.)
The work carried out jointly with Delft using careful X-ray examination showed that the Ni-alumina coprecipitate (tacovite) had the layered structure shown in Fig. 5 [10]. This consists of a series of brucite-like layers in which the Ni2+ and Al3+ ions of the precipitate are surrounded by hydroxyl ions and are separated by an interlayer containing water of crystallisation together with a charge-balancing anion to compensate for the excess charge introduced by the Al3+ species in the brucite layer. The balancing ion of the interlayer depends on the precipitant and the conditions of precipitation, simultaneous addition of the reactants at a carefully controlled constant pH giving more consistently reproducible results; at low pH values of about 5, the predominant ion was NO3− but at higher pH it was CO3=, the interlayer spacing being higher for the latter, and this result explained the differences in behaviours noted in the earlier work. The tacovite structure is an example of the more general mineral structure described as “hydrotalcite” and it was shown that is possible to incorporate different 2+ or 3+ ions in the structure. It is also possible to ion exchange such materials with other anionic species such as phosphate or molybdate. As an example, a coprecipitate containing Co2+ and Al3+ in the brucite-type layers was prepared and anion exchanged with molybdate species to give molybdate anions in the interlayer. The resultant material could be used for hyrodesulphurisation as effectively as a commercial impregnated catalyst of the type shown in Fig. 1. The proportion of 2+ to 3+ ions incorporated in the structure without any phase separation could also be varied over a wide range, from about 1 to about 6; however, this range decreased to 2 to 3 for hydrothermally aged samples. The activity and stability of the resultant catalysts tested in a conventional atmospheric pressure flow reactor with the catalyst bed at a temperature of 473 K was very dependent on all the steps in the preparation procedure, a particularly important one being the rate of heating during the calcination stage. Further work aimed at investigating the effect of various additives on the behaviour of these Ni-Al coprecipitates showed that all the Group 2 metal cations poisoned the activity of the catalysts while the addition of the Group I cations had little effect. However, it was found that the addition of La3+ species gave significant improvements of activity and stability, especially when the La3+ was added as nitrate during the coprecipitation step. It appeared that despite the fact that the La3+ ions are much larger than Ni2+ and Al3+, they were incorporated in the interlayers between the brucite layers of the coprecipitate [11, 12]. A patent from ICI from the same period also describes the preparation of coprecipitated catalysts incorporating other ions such as Mg2+ and Cr3+ [13]; these will have also been incorporated in the brucite structure by replacing some of the Ni2+ and Al3+ cations. A detailed review of some of the work that was carried out in Bradford and of some related work from the literature has been published elsewhere [14]. The topic of methanation catalysts has also been reviewed recently by Mebrahtu et al. [15]. A concept similar to the Adam and Eve project using solar power to bring about the steam reforming of methane and operated in combination with a methanation system has been described by Levy [16].
3 Development of a Novel Industrial Catalyst for Steam Reforming and Methanation
The work on lanthanum-containing catalysts was then expanded in a collaborative project with Dr. Rod Sambrook of Dyson Refractories Ltd. in Sheffield (hereafter referred to as Dysons). This company had for some time manufactured a catalyst developed by Nicklin et al. of British Gas [17, 18] that contained Ni and (depleted) urania supported on a porous alpha-alumina matrix in the form of Raschig rings. This was in use in a number of countries for the steam reforming of naphtha to produce “town gas”, a mixture of largely H2 and CO. A commercial problem with this very effective catalyst was that it had severe export restrictions because of the uranium content. As a result, Dysons were interested in finding a replacement catalyst. Within a relatively short period, we were able to develop a method to produce a catalyst that consisted of an active phase of Ni together with alumina and lanthana incorporated within the pores of the alpha-alumina Raschig rings, as shown schematically in Fig. 6. The preparation involved first the vacuum impregnation of pores of the alpha-alumina rings with a solution containing Ni, Al and La nitrates together with urea. The impregnated rings were then heated to a temperature just above 100 °C in order to hydrolyse the urea and to bring about precipitation of Ni, Al and La hydroxides in a brucite-type structure within the pores of the matrix. Excess water removed from the pores in the alpha alumina by careful heating and this step was followed by calcination at higher temperatures in order to decompose the hydroxide precipitate to the corresponding oxides.
The resultant catalysts were tested in Dysons’ laboratories for the steam reforming of a naphtha and they proved to be just as effective as the Ni-UO2-alumina materials discussed above. It was also found that the La-promoted catalyst had higher resistance to carbon deposition than an unpromoted material. It was shown that the La3+ ions were incorporated into the layer structure of the precipitate and that they helped to improve not only the stability of the resultant oxides but also the specific activity of the reduced catalysts [19]. Testing was therefore commenced under commercial conditions using customer facilities. One such test was particularly relevant. The catalyst was being operated in a pilot plant with a single tube reactor in parallel to a second single tube containing a commercial catalyst and using the same feed. The steam feed to both reactors was accidentally turned off. The bed containing the commercial catalyst became totally and permanently blocked as a result of carbon formation on the catalyst and this caused disintegration of the catalyst pellets. The Dyson catalyst remained intact, this being the result of the use of the strong alpha-alumina matrix. Its activity was then easily regenerated when the water feed was restored.
At about this time, this author paid a visit to the premises of Dublin Gas who were using the so-called cyclic reforming process to produce town gas from naphtha using another catalyst supplied by Dysons. The cyclic reforming process was carried out using a Munster Simms plant in which a layer of the catalyst was covered by another layer of alpha-alumina; this bed was heated to reaction temperature by a flame of the naphtha feed and then purged by steam before admitting the reaction mixture of naphtha and steam. The reaction then proceeded on the heated catalyst until the conversion dropped to too low a level at which stage the bed was again purged by steam before recommencing the cycle. The catalyst used consisted of Ni incorporated by impregnation into the pores of an alpha-alumina matrix. The engineer in charge of the plant described how the catalyst deactivated very rapidly and he produced some examples of spent catalyst rings; these exhibited the characteristic blue colouration of nickel aluminate (spinel). It required little persuasion to convince him that the Ni-Al-La-alpha-alumina catalyst might better withstand these conditions. Several months later Dublin Gas were provided with the first commercial charge of the new Dysons catalyst and started to use it very successfully for naphtha reforming. Shortly after this, the then recently commissioned Kinsale gas field off Cork started to produce natural gas and a pipeline was constructed to supply this gas to Dublin. During the period that it took to convert the appliances of all the domestic gas users to burn natural gas, Dublin Gas used their cyclic reforming plants, filled with the Dyson catalyst, to convert the Kinsale natural gas to a town gas. It transpired that the catalyst was so active that it was necessary to add additional natural gas to the reformat in order to obtain the required calorific value. Other commercial tests were also successful and the catalyst, which was patented internationally, became accepted for general use and was sold successfully internationally. A number of years later (1999), Dysons sold their catalyst business (then operating under the title Dycat) and their catalyst manufacturing plant to ICI who incorporated it into their newly formed Synetix Division. This whole division was then sold on to Johnson Matthey, its current owners, the sale being completed in 2005. It appears that the latter sale was part of a general consolidation of the catalyst manufacturing market and it would seem that Johnson Matthey has now probably ceased making the Dyson catalysts.
As part of the project on methanation catalysts in collaboration with Delft and Jülich described above (Sect. 2), the Dyson catalyst was also tested for the methanation reaction in a pilot plant designed to simulate the first bed of the Adam reactor combination shown in Fig. 4 and the results are shown in Fig. 7. The catalyst contained 6.4 wt.% Ni and the total bed length was 15 cm. The catalyst bed was preceded by 5 cm of inert packing (alpha alumina) to act as a preheater of the down-flow of reactant gas. The inlet temperature was held at about 230 °C and the exit temperature was limited to about 600 °C by some recirculation of the product gas. (The decrease in temperature which occurred down the bed after the maximum value was due to some heat loss to the surroundings.) The position of the high temperature peak moved slowly through the bed for the first 528 h, probably as a result of sintering of the catalyst, but then remained relatively stable for the remaining 480 hours of the test. Shortly after that, the Adam and Eve project was abandoned due to the German decision to cease using nuclear energy. As a result, the plant shown in Fig. 5 was operated for only a short time using a catalyst that was supplied by Haldor Topsoe. The energy transported between the steam reforming section and the methanation reactor was the equivalent to the energy requirements of a small town. A similar concept has been used to transmit energy from a solar concentrator supplying the heat required for the endothermic CO2 reforming of methane [14]. Methanation of CO2 using hydrogen produced by electrolysis using renewably generated electricity is a topic of current interest [15]; the methanation of CO2 generally proceeds more rapidly than that of CO but otherwise the catalysts used are the same and behave in exactly the same way. It is surprising that little reference is made in the current literature to the work that was carried out during the last century on the development of Ni methanation catalysts, an example of that work being that on coprecipitated Ni-alumina materials discussed in this article.
Data obtained for the methanation of CO in a single tube reactor simulating the reforming section of Fig. 4 showing the increase of temperature through the bed after various times of operation: (left to right) 24 h, 240 h, 528 h and 1008 h
Following the collaborative work with Delft, a new project on methanation catalysts was commenced with funding from the University of Twente to examine the effect of the Ni contents and promotion of the behaviour of coprecipitated catalysts. Each of the promoters tested (Ti, La, Ce) had a positive effect on the activities for methanation [20,21,22]. Increasing the Ni content also increased the ease of reduction of these catalysts as well as their activities without there being any affect on the existence of the tacovite layer structure in the catalyst precursors. However, the high temperature stabilities were decreased as a result of increasing the Ni contents, this having the consequence that these catalysts are probably more suitable for the methanation of low concentrations of CO (or CO2).
4 A Ni-Containing Catalyst for Methane Coupling
One of the main research interests in the catalysis group in Twente in the 1980’s, largely funded as part of several consecutive EU JOULE programmes, focussed on catalysts for the oxidative coupling of methane to form ethane and ethylene mixtures. Much of the work was devoted to work on materials such as Li on MgO which gave relatively high methane conversions and high selectivities to C2 products at temperatures above about 700 °C. Typical experiments using methane/oxygen ratios of about 5 gave up to 100% conversion of the O2 with selectivities and yields of C2 products of about 80% and 16% respectively. (The Li/MgO material was relatively unstable under operating conditions and it was shown that this instability was due to volatility of LiOH species.) We found that when a Li/MgO material was doped with SnO2, it gave good and relatively stable conversions and selectivities at temperatures below 700 °C [23]. It was well established that the methane coupling reaction operated on these types of material by a combination of catalytic and gas phase steps: the initiating step is the formation of methyl radicals on the catalyst surface and this is followed by a chain reaction involving the gas-phase methyl radicals and oxygen species. The consequence was that there is little chance of achieving any higher C2 yields unless a catalyst could be found that operates at temperatures below 700 °C, hence minimising the influence of the gas-phase chain reactions. It was recognised that only if C2 yields in excess of 20% could be achieved was the reaction likely to compete economically with the conventional route for ethylene production by hydrocarbon cracking. In 1990, Hans Heinemann and his coworkers published an intriguing paper in which they reported very high selectivities to C2 mixtures of greater than 90% from reaction at a temperature below 600 °C using a Ca3NiK0.1 oxide catalyst operating with 65% water in the feed [24]. This led us to investigate this system in some detail, the work being carried out by Kerry Dooley who was on a year’s sabbatical leave with us from the Louisiana State University. He found that the high selectivity reported by Heinemann was most probably due to the non-selective product of the reaction, CO2, reacting during the early stages of operation with the CaO of the catalyst; when this uptake of CO2 was complete, the selectivity decreased significantly [25]. (Heinemann also reported the same conclusion in a second paper published just before our report of this work appeared [26].) Kerry Dooley went on to show that it was possible to make a very stable catalyst of the composition 0.1 K/Ca/0.012 Ni that gave a yield of more than 20% to C2 products if water was present in the feed and that CO2 promoted the existence of a hydroxy-carbonate phase that gave selective conversion of methane at temperatures below 700 °C [27]. Some typical results are summarised in Table 1 where data are also given for some other similar catalyst formulations, including the Li/MgO/SnO2 catalyst discussed above. All the experiments were carried out in the presence of 16 mol% water with 30 mol% methane and 12 mol% oxygen, with a balance of helium; total pressure ca 1 atm. The temperatures giving the maximum yields were significantly lower than the temperatures require to give acceptable conversions and selectivities that had been reported in most other investigations of methane coupling carried out at that time. Baerns and Ross summarised some of the relevant literature on methane coupling in an article published in 1992 [28].
5 Nickel Catalysts for Internal Reforming in Molten Carbonate Fuel Cells
Two further research projects involving nickel catalysts were carried out in the Twente group. The first of these was funded by ECN (Energie Centrum Nederland) and concerned the use of Ni catalysts for the internal reforming of natural gas in a molten carbonate fuel cell. The principle behind such a fuel cell is that the hydrogen required for the operation of the fuel cell is formed by steam reforming using a catalyst placed in the assembly between successive fuel cell modules. The problem encountered in practice was that the Ni catalysts used gradually became poisoned by Li+ and K+ emanating from the molten salt mixture used in the cells, this arising from gas-phase transport of the alkali hydroxides to the catalyst surface. Experiments carried out using the assembly sketched in Fig. 8 showed that the K+ ions were transported more rapidly than the Li+ ions and that of a wide variety of Ni catalysts tested, coprecipitated materials with a Ni/Al ratio of 1:3 of the type described in Sect. 2 were the most resistant to poisoning [29].
6 Ni Catalysts for the CO2 (Dry) Reforming of Methane
The second Ni-related project followed on to the methane coupling work described above. Also funded under the EU JOULE programme, this involved the CO2 (“dry”) reforming of methane in what is again a thermodynamically reversible reaction:
The recurrent problem with catalysts for the dry reforming process, which would have to be carried out at temperatures above 800 °C to give useful conversions, is that the reaction occurs under conditions in which carbon deposition is thermodynamically favoured. Hence, most work on this topic in the literature has concentrated on trying to find catalysts which will minimise carbon formation under conditions when it is thermodynamically permitted. The dry reforming reaction provides a syngas mixture with a H2/CO ratio of 1.0 but this is too low for it to be used in reactions such as methanol synthesis or Fischer Tropsch synthesis. The problems of carbon deposition can be alleviated to some extent by adding either oxygen or steam to the feed and such additions also give a desirable increase of the H2/CO ratio in the product gases.
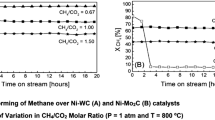
Our work showed that zirconia was a very promising support for both Ni or Pt catalysts for dry reforming. The experiments with Ni/ZrO2 demonstrated that a catalyst with low Ni loading (1–2%) was much more stable against carbon deposition than were samples with higher Ni loadings or a reference Ni/Al2O3 material [30]. An equivalent material containing Pt supported on zirconia was much more stable than the Ni-containing materials and, as a result, we continued for a number of years in Limerick to study the use of the Pt/ZrO2 catalysts, much of this work in a very successful collaboration with KTI BV (The Netherlands) [31], while the group in Twente, now under the leadership of Johannes Lercher, continued for a time to examine the Ni materials.
One further project on dry reforming using Ni catalysts ensued much later in Limerick. This was in a collaborative effort funded in an ERA Chemistry project and carried out together with Laszlo Guczi in Budapest and Alain Kienemann in Strasbourg. Our aim was to find a catalyst which resisted carbon deposition under dry reforming conditions. We found that a MgO–ZrO2 support prepared by coprecipitation (with a Mg/Zr molar ratio of 5:2) with a Ni loading of 8 wt% and gave a very good resistance to carbon deposition when promoted by 0.5 wt% K+ [32]; some representative results are given in Table 2. A patent application on these K-promoted catalysts has since been submitted, this being in cooperation with a Canadian company who have an interest in further developing the work [33]. It should be noted that this catalyst appears to be much more stable towards carbon deposition than the many other Ni-containing catalysts reported literature on the dry reforming reaction. Some of these papers have been summarised in a review of the subject [34]. As noted above, the dry reforming reaction gives a 1:1 ratio of hydrogen to CO whereas a 2:1 ratio would be required for reactions such as methanol synthesis or Fischer Tropsch synthesis purposes. It is therefore likely that water would be added to the feed in practice. Hence, the steam reforming reaction would be the primary reaction to be used, this being accompanied by the CO2 reforming reaction to give the required adjustment to the hydrogen/CO ratio. The catalyst would also need to be tested over much longer periods of operation under realistic conditions than is possible in a university research laboratory.
7 Brief Appreciation of Mike Spencer
It was with great sadness that I learnt of the death of Mike Spencer (Fig. 9), a scientific friend of many years standing. As has been described in detail elsewhere, Mike had two distinguished careers, the first of these as a researcher in what was then the Agricultural Division of ICI in Billingham, Teesside, and the second as an adjunct professor in Cardiff University. I was fortunate to have had extensive contact with him during both of these periods. I first met him as a result of regular visits to the University of Bradford by Mike and his colleagues from Billingham. It was through such contacts with industrial visitors such as Mike that the I learnt how important it is for an academic scientist working on catalysis to consider continuously the relevance and applicability of any research carried out. It was quite clear that there is little point in preparing and testing a catalyst under conditions that would not be relevant in practice.
At a later stage, when still in Bradford, I became Editor of the News Brief section of the then new journal Applied Catalysis and I invited Mike to become a member of a team of “correspondents” for that section. For many years after that, Mike provided me a wide range of very valuable and meticulously argued contributions, many of these relating to his favourite topic, copper catalysts for methanol synthesis. Mike’s thought-provoking items for News Brief continued after he had retired from ICI and moved to Cardiff. By then, I had moved to my position in the University of Twente and Mike visited my research group there, giving a series of lectures to members of my group as well as to invited guests from outside the University. When, later still in 1991, I moved to the University of Limerick, this practice continued and we continued a regular correspondence until very recently. He will be sadly missed by all those who knew him and have benefitted from his wise counsel.
8 Conclusions
The intention of this review is to show that it is possible for an academic group to carry out a mixture of focussed academic and applied work. In all the projects described, this aim was facilitated by developing close links with other groups and often also with industrial partners. While it is generally not possible in an academic laboratory to test catalysts under conditions fully consistent with industrial requirements, it was generally possible to obtain results of relevance to industrial applications. The author has benefitted greatly throughout his career from discussions with a number of prominent industrial scientists, Mike Spencer being foremost among them, who were able to provide broad views of the whole field of catalysis and the relevant literature and to help him remain focussed on possible applications of his work. The author would encourage younger academics who are developing an interest in the catalytic aspects of C1 chemistry, or indeed other aspects of applied catalysis, to establish scientific links such as those described above and always to keep an eye on the practical requirements of the catalyst system they are investigating. He would also encourage them not to ignore some of the extensive older literature on the subject. Finally, he would like to emphasise that an approach of the type described also has the potential of offering great benefits in terms of career prospects to the students and postdoctoral workers involved in the projects.
References
Ross JRH, Steel MCF (1973) Mechanism of the steam reforming of methane over a coprecipitated nickel-alumina catalyst. J Chem Soc Faraday Trans I 69:10–20
Ridler DE, Twigg MV (1989) Steam reforming. In: Twigg MV (ed) Catalyst handbook, 2nd edn. CRC Press, Taylor & Francis Group, London/New York, pp 225–282
Bodrov NM, Apel’baum LOMI, Tempkin (1964) Kinet Catal. 5:614
Ross JRH, Steel MCF, Zeini-Isfahani A (1978) Evidence for the participation of surface nickel aluminate sites in the steam reforming of methane over nickel/alumina catalysts. J Catal 52:280–290
Ross JRH (1975) The Steam Reforming Of Hydrocarbons. In: Roberts MW, Thomas JM (eds) Surface and defect properties of solids, vol. 4. Royal Society of Chemistry, London, pp 34–67
Beecroft T, Millar AW, Ross JRH (1975) The use of differential scanning calorimetry in catalyst studies. The methanation of carbon monoxide over nickel/alumina catalysts. J Catal 40:281–285
Broecker FU, Dethlefsen W, Kaempfer K, Marosi L, Schwarzmann M, Triebskorn B, Zirker G (1974) German Patent 2,255,877
Hoehlein B, Menzer R, Range J (1981) High temperature methanation in the long-distance energy transport system. Appl Catal 1:125–139
Pearce BB, Twigg MV, Woodward C (1989) Methanation. In: Twigg MV (ed) Catalyst handbook, 2nd edn. CRC Press/Taylor & Francis Group, London/New York, pp 340–383
Kruissink Edgar C, van Reijen Louis L, Ross Julian R.H. (1981) Coprecipitated nickel-alumina catalysts for methanation at high temperature - part 1 chemical composition and structure of the precipitates. J Chem Soc Faraday Trans I 77:649–663
Gelsthorpe MR, Mok KB, Ross JRH, Sambrook RM (1984) The effect of lanthanum additives on the catalytic activities of Ni-Al2O3 coprecipitated catalysts for the methanation of carbon monoxide. J Mol Catal 25:253–262
Sambrook RM, Ross JRH (1984) Catalyst and Method of Preparation. U.S. Patent 4,469,815
Gent CW, Assets JR, Farmers K, Woodward C (1978) Methanation. British Patent 1(504):866
Ross JRH (1985) Metal catalysed methanation and steam reforming. In: Bond GC, Webb G (eds) Catalysis, vol 4. Royal Society of Chemistry, London, pp 1–45
Mebrahtu C, Krebs F, Abate S, Perathoner S, Centi G, Palkovits R (2019) CO2 Methanation: principles and challenges. Stud Surf Sci Catal 178:85–103
Levy D (1993) Solar Energy 50:179–189
Nicklin T, Whittaker RJ (1968) Inst. Gas Eng. J. 8:15
Niklin T, Farrington F (1970) Inst Gas Eng J 13:151
Mok KB, Ross JRH, Sambrook RM (1983) Thermally and mechanically stable catalysts for steam reforming and methanation. A new concept in catalyst design. In: Poncelet G, Grange P, Jacobs PA (eds) Preparation of catalysts III. studies in surface science and catalysis, vol 16. Elsevier, New York, pp 291–299
Lansink Rotgerink HGH, Mercera PDL, van Ommen JG, Ross JRH (1988) Studies on promotion of nickel-alumina coprecipitated catalysts: titanium oxide. Appl Catal 45:239–256
Lansink Rotgerink HGH, Paalman RPAM, van Ommen JG, Ross JRH (1988) Studies on promotion of nickel-alumina coprecipitated catalysts: lanthanum oxide. Appl Catal 45:257–280
Lansink Rotgerink HGH, Slaa JC, van Ommen JG, Ross JRH (1988) Studies on promotion of nickel-alumina coprecipitated catalysts: cerium oxide. Appl Catal 45:281–290
Korf SJ, Roos JA, Ross JRH (1992) The development of doped Li/MgO catalyst systems for the low-temperature oxidative coupling of methane. In: Wolf EE, Van Nostrand D (eds) Methane conversion by oxidative processes: fundamentals and engineering aspects. Reinhold, New York, pp 168–199
Pereira P, Lee SH, Somorjai GA, Heinemann H (1990) Catal Lett 6:255
Dooley KM, Ross JRH (1992) Potassium/calcium/nickel oxide catalysts for oxidative coupling of methane. Appl Catal A 90:159–174
Rasco J, Somorjai GA, Heinemann H (1992) Appl Catal A 84:57–75
Dooley KM, Chen S-Y, Ross JRH (1994) Stable nickel-containing catalysts for the oxidative coupling of methane. J Catal 145:402–408
Baerns M, Ross JRH (1992) Catalytic chemistry of methane conversion. In: Thomas JM, Zamaraev KI (eds) Perspectives in catalysis, IUPAC. Blackwell Science, Hoboken, pp 315–335
Berger RJ, Doesburg EBM, van Ommen JG, Ross JRH (1994) Deactivation behaviour of nickel catalysts used for internal reforming in molten carbonate fuel cells. Stud Surf Sci Catal 81:309–314
Seshan K, ten Barge HW, Hally W, van Keulen ANJ, Ross JRH (1994) Carbon dioxide reforming of methane in the presence of nickel and platinum catalysts. Stud Surf Sci Catal 81:285–290
van Keulen ANJ, Hegarty MES, Ross JRH, van den Oosterkamp P (1997) The development of platinum-zirconia catalysts for the CO2 reforming of methane. Stud Surf Sci Catal 107:537–548
Nagaraja BM, Bulushev DA, Beloshapkin S, Ross JRH (2011) The effect of potassium on the activity and stability of Ni-MgO-ZrO2 catalysts for the dry reforming of methane to give synthesis gas. Catal Today 178:132–136
Ross JRH, Nagaraja BM, Bulushev DA (2015) Potassium-doped Ni-MgO-ZrO2 catalysts for dry reforming of methane to synthesis gas. U.S. Patent Application, 14/754,670
Ross JRH (2014) Syngas production using carbon dioxide reforming: fundamentals and perspectives. In: Bhanage BM, Arai M (eds) Transformation and utilisation of carbon dioxide. Springer-Verlag, Berlin, Heidelberg, pp 131–161
Acknowledgements
The author would like to acknowledge with thanks the contributions of the many researchers who worked with him during the period described in this review as well a large variety of different funders, too many to list here. Most of the researchers and funders are mentioned where appropriate in the text. He would also like to thank Martyn Twigg for having encouraged him to write this review and the referees for their very helpful and constructive suggestions for improvements.
Funding
Open Access funding provided by the IReL Consortium. The work discussed in this review paper was funded by a wide variety of organisations and these are mentioned where appropriate in the text. None of this made any stipulation regarding publication of the work and so the author is free to publish all of the information given in the text.
Author information
Authors and Affiliations
Contributions
The author is responsible for all the content but he acknowledges the contributions of the many researchers who worked with him during the period described in this review, too many to list here. Most of them are mentioned where appropriate in the text.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ross, J.R.H. Nickel Catalysts for C1 Reactions: Recollections from a Career in Heterogeneous Catalysis. Top Catal 64, 896–906 (2021). https://doi.org/10.1007/s11244-021-01433-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01433-0