Abstract
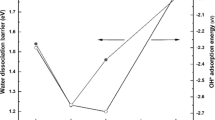
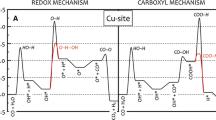
Using the density functional theory, the mechanism of the water–gas shift reaction has been investigated employing a model catalyst formed by a Au5 cluster supported on the Fe-terminated (0001) face of hematite (α-Fe2O3), to better understand the role played by the metal–oxide interface in this reaction. Our results indicate that the Au5/hematite model catalyst has a good performance to catalyze the reaction following the so-called adsorptive mechanism. The presence of Au favors the development of the reaction due mainly to the following factors: (i) H2O dissociates very easily at the metal–oxide interface producing OH species; (ii) CO adsorbs strongly on a Au site nearby the position of OH; (iii) the hydroxycarbonyl intermediate (HOCO) is formed at the interface from CO and OH with a low activation barrier; and (iv) after hydrogen releasing, CO2 is desorbed with relative facility from the interface region. The formation of H2 is the stage of the whole reaction that more energy demands; however, this process is favored if one hydrogen atom comes directly from HOCO, instead of from two hydrogen atoms bound to surface oxygen anions.






Similar content being viewed by others
References
Pal DB, Chand R, Upadhyay SN, Mishra PK (2018) Performance of water gas shift reaction catalysts: a review. Renew Sustain Energy Rev 93:549–565
Hong YK, Lee DW, Ko YC, Yinghua L, Han HS, Lee KY (2010) Passive NOx reduction with CO using Pd/TiO2/Al2O3 + WGSR catalysts under simulated post-Euro IV diesel exhaust conditions. Catal Lett 136:106–115
Hu YH, Ruckenstein E (2002) Binary MgO-based solid solution catalysts for methane conversion to syngas. Catal Rev Sci Eng 44:423–453
Silberova BAA, Mul G, Makkee M, Moulijn JA (2006) DRIFTS study of the water–gas shift reaction over Au/Fe2O3. J Catal 243:171–182
Haruta M, Kobayashi T, Sano H, Yamada N (1987) Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem Lett 16:405–408
Landon P, Ferguson J, Solsona BE, Garcia T, Carley AF, Herzing AA, Kiely CJ, Golunski SE, Hutchings GJ (2005) Selective oxidation of CO in the presence of H2, H2O and CO2 via gold for use in fuel cells. Chem Commun 27:3385–3387
Andreeva D, Idakiev V, Tabakova T, Andreev A, Giovanoli R (1996) Low-temperature water–gas shift reaction on Au/α-Fe2O3 catalyst. Appl Catal A 134:275–283
Andreeva D (2002) Low temperature water gas shift over gold catalysts. Gold Bull 35:82–88
Venugopal A, Scurrell MS (2004) Low temperature reductive pretreatment of Au/Fe2O3 catalysts, TPR/TPO studies and behavior in the water–gas shift reaction. Appl Catal A 258:241–249
Kudo S, Maki T, Fukuda T, Mae K (2011) Pre-reduction of Au/iron oxide catalyst for low-temperature water-gas shift reaction below 150°C. Catalysts 1:175–190
Soria MA, Pérez P, Carabineiro SAC, Maldonado-Hódar FJ, Mendes A, Madeira LM (2014) Effect of the preparation method on the catalytic activity and stability of Au/Fe2O3 catalysts in the low-temperature water-gas shift reaction. Appl Catal A 470:45–55
Luengnaruemitchai A, Osuwan S, Gulari E (2003) Comparative studies of low-temperature water-gas shift reaction over Pt/CeO2, Au/CeO2 and Au/Fe2O3 catalysts. Catal Commun 4:215–221
Bocuzzi F, Chiorino A, Manzoli M, Andreeva D, Tabakova T (1999) FTIR study of the low-temperature water-gas shift reaction on Au/Fe2O3 and Au/TiO2 catalysts. J Catal 188:176–185
Deng W, Carpenter C, Yi N, Flytzani-Stephanopoulos M (2007) Comparison of the activity of Au/CeO2 and Au/Fe2O3 catalysts for the CO oxidation and the water-gas shift reactions. Top Catal 44:199–208
Daniells ST, Makkee M, Moulijn JA (2005) The effect of high-temperature pre-treatment and water on the low temperature CO oxidation with Au/Fe2O3 catalysts. Catal Lett 100:39–47
Jiying W, Zhenzhong Z, Lan Z, Bifang M, Feng J (2012) Analysis or the active Au species on Au/Fe2O3 catalyst. Rare Met Mater Eng 41:377–382
Minicò S, Scirè S, Crisafulli C, Visco AM, Galvagno S (1997) FT-IR study of Au/Fe2O3 catalysts for CO oxidation at low temperature. Catal Lett 47:273–276
Hodge NA, Kiely CJ, Whyman R, Siddiqui MRH, Hutchings GJ, Pankhurst QA, Wagner FE, Rajaram RR, Golunski SE (2002) Microstructural comparison of calcined and uncalcined gold/iron-oxide catalysts for low-temperature CO oxidation. Catal Today 72:133–144
Gokhale AA, Dumesic JA, Mavrikakis M (2008) On the mechanism of low-temperature water gas shift reaction on copper. J Am Chem Soc 130:1402–1414
Fajín JLC, Cordeiro MDS, Illas F, Gomes JRB (2009) Influence of step sites in the molecular mechanism of the water gas shiftreaction catalyzed by copper. J Catal 268:131–141
Wong K, Zeng QH, Yu AB (2011) Electronic structure of metal (M = Au, Pt, Pd, or Ru) bilayer modified & α-Fe2O3(0001) surfaces. J Phys Chem C 115:4656–4663
Kiejna A, Pabisiak T (2012) Surface properties of clean and Au or Pd covered hematite (α-Fe2O3) (0001). J Phys Condens Matter 24:095003
Pabisiak T, Winiarski MJ, Kiejna A (2016) CO adsorption on small Aun (n = 1–4) structures supported on hematite. I. Adsorption on iron terminated α-Fe2O3 (0001) surface. J Chem Phys 144:044704
Pabisiak T, Winiarski MJ, Kiejna A (2016) CO adsorption on small Aun (n = 1–4) structures supported on hematite. II. Adsorption on the O-rich termination of α-Fe2O3(0001) surface. J Chem Phys 144:044705
Nguyen MT, Farnesi Camellone M, Gebauer R (2015) On the electronic, structural, and thermodynamic properties of Au supported on α-Fe2O3 surfaces and their interaction with CO. J Chem Phys 143:034704
Howard KL, Willock DJ (2011) A periodic DFT study of the activation of O2 by Au nanoparticles on α-Fe2O3. Faraday Discuss 152:135–151
Fuente SA, Fortunato LF, Zubieta C, Ferullo RM, Belelli PG (2018) Water dissociation at the Au/α-Fe2O3(0001) interface. Mol Catal 446:10–22
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences, and uses. Wiley-VCH, Weinheim
Thevuthasana S, Kim YJ, Yi SI, Chambers SA, Morais J, Denecke R, Fadley CS, Liuc P, Kendelewicz T, Brown GE Jr (1999) Surface structure of MBE-grown α-Fe2O3(0001) by intermediate-energy X-ray photoelectron diffraction. Surf Sci 425:276–286
Chambers SA, Fe Yi SI (1999) termination for α-Fe2O3(0001) as grown by oxygen-plasma-assisted molecular beam epitaxy. Surf Sci Lett 439:L785–L791
Shaikhutdinov SK, Weiss W (1999) Oxygen pressure dependence of the α-Fe2O3(0001) surface structure. Surf Sci Lett 432:L627–L634
Wang XG, Weiss W, Shaikhutdinov SK, Ritter M, Peterson M, Wagner F, Schlögl R, Scheffler M (1998) The hematite (α-Fe2O3) (0001) surface: evidence for domains of distinct chemistry. Phys Rev Lett 81:1038–1041
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47:558–561
Kresse G, Hafner J (1993) Ab initio molecular dynamics for open-shell transition metals. Phys Rev B 48:13115–13118
Kresse G, Hafner J (1994) Ab initio molecular-dynamics simulation of the liquid–metal-amorphous-semiconductor transition in germanium. Phys Rev B 49:14251–14269
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46:6671–6687
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1993) Erratum: atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 48:4978
Plata JJ, Graciani J, Evans J, Rodriguez JA, Fernández Sanz J (2016) Cu deposited on CeOx-modified TiO2(110): synergistic effects at the metal–oxide interface and the mechanism of the WGS reaction. ACS Catal 6:4608–4615
Rodríguez JA, Evans J, Graciani J, Park JB, Liu P, Hrbek J, Fdez Sanz J (2009) High water-gas shift activity in TiO2(110) supported Cu and Au nanoparticles: role of the oxide and metal particle size. J Phys Chem C 113:7364–7370
Peng SF, Ho JJ (2011) The mechanism of the water-gas shift reaction on Cu/TiO2(110) elucidated from application of density-functional theory. Phys Chem Chem Phys 13:20393–20400
Mudiyanselage K, Senanayake SD, Feria L, Kundu S, Baber AE, Graciani J, Vidal AB, Agnoli S, Evans J, Chang R, Axnanda S, Liu Z, Sanz JF, Liu P, Rodriguez JA, Stacchiola DJ (2013) Importance of the metal–oxide interface in catalysis. In situ studies of the water-gas shift reaction by ambient-pressure X-ray photoelectron spectroscopy. Angew Chem Int Ed 52:5101–5105
Blochl P (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775
Rollmann G, Rohrbach A, Entel P, Hafner J (2004) First-principles calculation of the structure and magnetic phases of hematite. Phys Rev B 69:165107
Finger LW, Hazen RM (1980) Crystal structure and isothermal compression of Fe2O3, Cr2O3, and V2O3 to 50 Kbars. J Appl Phys 51:5362
Coey J, Sawatzky G (1971) A study of hyperfine interactions in the system (Fe1−xRhx)2O3 using the Mossbauer effect (bonding parameters). J Phys C 4:2386–2407
Yang CT, Wood BC, Bhethanabotla VR, Joseph B (2015) The effect of themorphology of supported subnanometer Pt clusters on the first and key step of CO2 photoreduction. Phys Chem Chem Phys 17:25379–25392
Barrio L, Liu P, Rodríguez JA, Campos-Martin JM, Fierro JLG (2006) A density functional theory study of the dissociation of H2 on gold clusters: importance of fluxionality and ensemble effects. J Chem Phys 125:164715
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford Science, Oxford
Shubina TE, Hartnig C, Koper MTM (2004) Density functional theory study of the oxidation of CO by OH on Au(110) and Pt(111) surfaces. Phys Chem Chem Phys 6:4215–4221
Plata JJ, Romero-Sarria F, Amaya Suárez J, Márquez AM, Laguna OH, Odriozola JA, Sanz JF (2018) Improving the activity of gold nanoparticles for the water gas shift reaction using TiO2-Y2O3: an example of catalysts design. Phys Chem Chem Phys 20:22076–22083
Liu ZP, Jenkins SJ, King DA (2005) Origin and activity of oxidized gold in water-gas-shift catalysis. Phys Rev Lett 94:196102
Chiang HN, Jiang JC (2013) Density functional theory study of water-gas-shift reaction on 3Cu/α-Al2O3(0001) surface. J Phys Chem C 117:12045–12053
Campbell CT, Sellers JRV (2012) The entropies of adsorbed molecules. J Am Chem Soc 134:18109–18115
He Y, Liu JC, Luo L, Wang YG, Zhu J, Du Y, Li J, Mao SX, Wang C (2018) Size-dependent dynamic structures of supported gold nanoparticles in CO oxidation reaction condition. Proc Natl Acad Sci USA 115:7700–7705
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397
Acknowledgements
Authors thank Universidad Nacional del Sur (UNS), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) of Argentina for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fuente, S.A., Zubieta, C., Ferullo, R.M. et al. Theoretical Study of the Water–Gas Shift Reaction on a Au/Hematite Model Catalyst. Top Catal 62, 908–917 (2019). https://doi.org/10.1007/s11244-019-01174-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-019-01174-1