Abstract
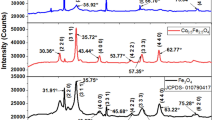
CeFex (x = 0, 1, 3, 5, 10, 15 and 20 at.%) mixed oxides synthesized by an adapted Pechini method were characterized by Raman spectroscopy, high-resolution transmission electron microscopy, electron paramagnetic resonance, magnetization and 57Fe Mössbauer spectroscopy (57Fe-MS) measurements in order to evaluate the oxygen vacancies formation and the chemical environment of Fe+3 inserted into the CeO2 crystalline lattice. Fe+3 introduction into the CeO2 structure resulted in an increase of the oxygen vacancies concentration, which indicates that this is the predominant charge compensation mechanism in the formation of CeFex solid solutions by the Pechini method. Fe+3 insertion in CeO2 led to the formation of substitutional solid solutions, in which Fe+3 replaced octahedral Ce+4 sites in the crystalline lattice. Fe+3 could be found in the form of isolated sites with orthorhombic distortion or Fe+3 species in pairs or clusters coupled by strong spin–spin interactions. No evidence of Fe+3 insertion into tetrahedral interstitial sites was found. Isolated Fe+3 species showed a less distorted chemical environment and greater ionic character of the Fe–O bonds than the clusters, being the concentration of both type sites approximately equal for all the Fe+3 doped contents. It was found that pure CeO2 and all the CeFex mixed oxides presented ferromagnetic properties even at room temperature possibly due to their small crystallite size and the presence of oxygen vacancies. At high Fe+3 concentrations (above 10 at.%), probably super-exchange interactions (Fe+3–O−2–Fe+3), with an antiferromagnetic character, also took place, reducing the ferromagnetism of the CeFex mixed oxides.











Similar content being viewed by others
References
Trovarelli A (2002) Catalysis by ceria and related materials (1 ed.), Vol. 2, Imperial College Press, London
Aneggi E, Boaro M, de Leitenburg C, Dolcetti G, Trovarelli A (2006) J Alloy Compd 408–412:1096–1102
Mukherjee D, Rao BG, Reddy BM (2017) Top Catal 60:1673–1681
Laguna OH, Centeno MA, Boutonnet M, Odriozola JA (2011) Appl Catal B 106:621–629
Wang J, Zhang B, Shen M, Wang J, Wang W, Ma J, Liu S, Jia L (2011) J Sol-Gel Sci Technol 58:259–268
Trovarelli A, de Leitenburg C, Boaro M, Dolcetti G (1999) Catal Today 50:353–367
Yao X, Tang C, Ji Z, Dai Y, Cao Y, Gao F, Dong L, Chen Y (2013) Catal Sci Technol 3:688–698
Wang J, Shen M, Wang J, Cui M, Gao J, Ma J, Liu S (2012) J Environ Sci 24:757–764
Bao H, Chen X, Fang J, Jiang Z, Huang W (2008) Catal Lett 125:160–167
Bao H, Qian K, Fang J, Huang W (2017) Appl Surf Sci 414:131–139
Luo Y, Chen R, Peng W, Tang G, Gao X (2017) Appl Surf Sci 416:911–917
Qiao D, Lu G, Liu X, Guo Y, Wang Y, Guo Y (2011) J Mater Sci 46:3500–3506
Li K, Wang H, Wei Y, Liu M (2008) J Rare Earths 26:245–249
Hong W-J, Ueda M, Iwamoto S, Hosokawa S, Wada K, Kanai H, Deguchi H, Inoue M (2011) Appl Catal B 106:142–148
Sahoo TR, Armandi M, Arletti R, Piumetti M, Bensaid S, Manzoli M, Panda SR, Bonelli B (2017) Appl Catal B 211:31–45
Gu Z, Li K, Wang H, Wei Y, Yan D, Qiao T (2013) Kinet Catal 54:326–333
Wang W, Zhu Q, Qin F, Dai Q, Wang X (2018) Chem Eng J 333:226–239
Ilieva L, Pantaleo G, Velinov N, Tabakova T, Petrova P, Ivanov I, Avdeev G, Paneva D, Venezia AM (2015) Appl Catal B 174–175:176–184
Wang Y, Wang F, Chen Y, Zhang D, Li B, Kang S, Li X, Cui L (2014) Appl Catal B 147:602–609
Brackmann R, Toniolo FS, Schmal M (2016) Top Catal 59:1772–1786
Lagarec K, Rancourt DG (1997) Nucl Instrum Methods Phys Res Sect B 129:266–280
Dunlap RA, McGraw JD (2007) J Non-Cryst Solids 353:22–23
Rossano S, Balan E, Morin G, Bauer J-P, Calas G, Brouder C (1999) Phys Chem Miner 26:530–538
Dunlap RA, Sibley ADE (2004) J Non-Cryst Solids 337:36–41
McBride JR, Hass KC, Poindexter BD, Weber WH (1994) J Appl Phys 76:2435–2441
Shen Q, Lu G, Du C, Guo Y, Wang Y, Guo Y, Gong X (2013) Chem Eng J 218:164–172
Ilieva L, Pantaleo G, Ivanov I, Venezia AM, Andreeva D (2006) Appl Catal B 65:101–109
Minervini L, Zacate MO, Grimes RW (1999) Solid State Ionics 116:339–349
Moog I, Feral-Martin C, Duttine M, Wattiaux A, Prestipino C, Figueroa S, Majimel J, Demourgues A (2014) J Mater Chem A 2:20402–20414
Zhang Z, Han D, Wei S, Zhang Y (2010) J Catal 276:16–23
Liang C, Ma Z, Lin H, Ding L, Qiu J, Frandsen W, Su D (2009) J Mater Chem 19:1417–1424
Skorodumova NV, Simak SI, Lundqvist BI, Abrikosov IA, Johansson B (2002) Phys Rev Lett 89:166601-1–166601-4
Venkatesan M, Fitzgerald CB, Coey JMD (2004) Nature 430:630
Liu Y, Lockman Z, Aziz A, MacManus-Driscoll J (2008) J Phys: Condens Matter 20:165201–165205
Sharma SK, Knobel M, Meneses CT, Kumar S, Kim YJ, Koo BH, Lee CG, Shukla DK (2009) J Korean Phys Soc 55:1018–1021
Paunović N, Dohčević-Mitrović Z, Scurtu R, Aškrabić S, Prekajski M, Matović B, Popović ZV (2012) Nanoscale 4:5469–5476
Radović M, Dohčević-Mitrović Z, Paunović N, Šćepanović M, Matović B, Popović ZV (2009) Acta Phys Pol A 116:84–87
Sundaresan A, Bhargavi R, Rangarajan N, Siddesh U, Rao CNR (2006) Phys Rev B 74:161306-1–161306-4
Wang W-C, Chen S-Y, Glans P-A, Guo J, Chen R-J, Fong K-W, Chen C-L, Gloter A, Chang C-L, Chan T-S, Chen J-M, Lee J-F, Dong C-L (2013) Phys Chem Chem Phys 15:14701–14707
Coey JMD, Douvalis AP, Fitzgerald CB, Venkatesan M (2004) Appl Phys Lett 84:1332–1334
Phokha S, Pinitsoontorn S, Maensiri S (2013) Nano-Micro Lett 5:223–233
Verma KC, Singh J, Ram M, Sharma DK, Sharma A, Kotnala RK (2012) Phys Scr 86:025704–025711
Acknowledgements
The authors thank the Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) and the National Counsel of Technological and Scientific Development (CNPq) for financial support (scholarship). We thank Rosa B. Scorzelli for made available the Mössbauer facility at Centro Brasileiro de Pesquisas Físicas (CBPF)—Brazil. The authors also acknowledge Núcleo de Microscopia da COPPE-UFRJ for the use of the facilities.
Funding
Funding was provided by Universidade Federal do Rio de Janeiro.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brackmann, R., Toniolo, F.S., dos Santos Filho, E. et al. Characterization of CeO2–Fe2O3 Mixed Oxides: Influence of the Dopant on the Structure. Top Catal 61, 1694–1706 (2018). https://doi.org/10.1007/s11244-018-1031-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-1031-1