Abstract
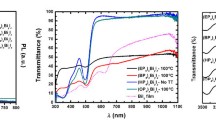
In recent times, halide perovskites have attracted the attention of the scientific community because of their semiconducting properties, low cost, and easy synthesis methods; characteristics that make them suitable for application as solar cells and in other fields of electronics. However, these materials are subject to significant chemical and structural degradation and performance decrease over time. This degradation remains a critical factor that prevents their widespread use. In the present paper, we investigated the stability of different bromine-based perovskites: hybrid, MAPbBr3 (MA:NH3CH3+) and inorganic, CsPbBr3 and RbPbBr3. The samples were prepared via single-step solution synthesis as thin films on Au/Pd coated glass substrates and the ageing process was carried out in air within a climatic chamber maintained at 50 °C and 75%RH humidity. The structural and chemical evolution of the perovskites was evaluated as a function of the exposure time by means of AFM, and AR-XPS, while their optoelectronic properties were monitored by indirect bandgap measurement. We observed that the inorganic perovskites resulted more stable than mixed organic–inorganic perovskites. Nevertheless, degradation remains present and further studies are necessary if these materials are to be used for practical applications.




Similar content being viewed by others
References
Chu S, Majumdar A (2012) Opportunities and challenges for a sustainable energy future. Nature 488(7411):294–303
O’Regan B, Gratzel M (1991) A low-cost high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346):737–740
Zhu X (2016) The perovskite fever and beyond. ACC Chem Res 49(3):355–356
Liu X et al (2015) Organic–inorganic halide perovskite based solar cells—revolutionary progress in photovoltaics. Inorg Chem Front 2:315–335
Green MA, Emery K, Hishikawa Y, Warta W, Dunlop ED (2015) Solar cell efficiency tables (version 46). Prog Photovol Res Appl 23(7):805–812
Oku T (2015) Crystal structures of CH3NH3PbI3 and related perovskite compounds used for solar cells. In: Kosyachenko LA (ed) Solar cells. IntechOpen. Available from https://www.intechopen.com/books/solar-cells-new-approaches-and-reviews/crystal-structures-of-ch3nh3pbi3-and-related-perovskite-compounds-used-for-solar-cells. Accessed 19 Apr 2018
Chen W et al (2015) Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 350(6263):944–948
Grätzel M (2014) The light and shade of perovskite solar cells. Nat Mater 13(9):838–842
Park N-G, Grätzel M, Miyasaka T, Zhu K, Emery K (2016) Towards stable and commercially available perovskite solar cells. Nat Energy 1:16152
Hodes G (2013) Perovskite-based solar cells. Science 342(6156):317–318
Domanski K et al (2016) Not all that glitters is gold: metal migration-induced degradation in perovskite solar cells. ACS Nano 10(6):6306–6314
Xiao Z et al (2016) Thin-film semiconductor perspective of organometal trihalide perovskite materials for high-efficiency solar cells. Mater Sci Eng R Rep 101:1–38
Chen Q et al (2015) Under the spotlight: the organic-inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 10(3):355–396
Lee YH, Luo J, Humphry-Baker R, Gao P, Grätzel M, Nazeeruddin MK (2015) Unraveling the reasons for efficiency loss in perovskite solar cells. Adv Funct Mater 25(25):3925–3933
Leguy AMA et al (2015) Reversible hydration of CH3NH3PbI3 in films, single crystals, and solar cells. Chem Mater 27(9):3397–3407
Misra RK et al (2015) Temperature- and component-dependent degradation of perovskite photovoltaic materials under concentrated sunlight. J Phys Chem Lett 6(3):326–330
Xu T, Chen L, Guo Z, Ma T (2016) Themed issue: physical chemistry of hybrid perovskite solar cells strategic improvement of the long-term stability of perovskite materials and perovskite solar cells. Phys Chem Chem Phys 18(18):27026–27050
Ong KP, Goh TW, Xu Q, Huan A (2015) Structural evolution in methylammonium lead iodide CH3NH3PbI3. J Phys Chem A 119(44):11033–11038
R. Gottesman et al (2015) Photoinduced reversible structural transformations in free-standing CH3NH3PbI3 perovskite films. J Phys Chem Lett 6(12):2332–2338
Han Y et al (2015) Degradation observations of encapsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J Mater Chem A 3(15):8139–8147
Cui J et al (2015) Recent progress in efficient hybrid lead halide perovskite solar cells. Sci Technol Adv Mater 16(3):36004
Shirley DA (1972) High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys Rev B 5(12):4709–4714
Smith GC, Seah MP (1990) Standard reference spectra for XPS and AES: their derivation, validation and use. Surf Interface Anal 16(1–12):144–148
Christians JA, Miranda Herrera PA, Kamat PV (2015) Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J Am Chem Soc 137:1530–1538
Clegg C, Hill IG (2016) Systematic study on the impact of water on the performance and stability of perovskite solar cells. RSC Adv 6:52448–52458
Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions. J Res Natl Bur Stand A 81(1):89–96
Boldish SI, White WB (1998) Optical band gaps of selected ternary sulfide minerals. Am Mineral 83(7–8):865–871
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi 15:627–637
Giaccherini A et al (2016) Green synthesis of pyrite nanoparticles for energy conversion and storage: a spectroscopic investigation. Eur J Mineral 28(3):611–618
Paynter RW, Nolet D (2003) Parametric analysis of the extraction of depth profile information from ARXPS data obtained on a silicon wafer sample. Surf Interface Anal 35:960–967
Choi J, Zhang J, Liou S-H, Dowben PA, Plummer EW (1999) Surfaces of the perovskite manganites La1−xCaxMnO3. Phys Rev B 59(20):13453
Guo X, McCleese C, Kolodziej C, Samia ACS, Zhao Y, Burda C (2016) Identification and characterization of the intermediate phase in hybrid organic-inorganic MAPbI3 perovskite. Dalton Trans 45(9):3806–3813
Shahbazi M, Wang H (2016) Progress in research on the stability of organometal perovskite solar cells. Sol Energy 123:74–87
Huang S et al (2017) Morphology evolution and degradation of CsPbBr3 nanocrystals under blue ligh-emitting diode illumination. ACS Appl Mater Interfaces 9:7249–7258
Chen J et al (2016) Photo-stability of CsPbBr3 perovskite quantum dots for optoelectronic application. Sci China Mater 59:719–727
Acknowledgements
Project co-financed under Tuscany POR FESR 2014–2020 ((Bando 2) project name: “SpettroX”). N.C. and S.C. acknowledge the financial support provided by Ente Cassa di Risparmio di Firenze (Project 2015.0937).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calisi, N., Caporali, S., Milanesi, A. et al. Composition-Dependent Degradation of Hybrid and Inorganic Lead Perovskites in Ambient Conditions. Top Catal 61, 1201–1208 (2018). https://doi.org/10.1007/s11244-018-0922-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-018-0922-5