Abstract
A combined spectroscopic and computational study of gas-phase Au+(CH4) n (n = 3–8) complexes reveals a strongly-bound linear Au+(CH4)2 core structure to which up to four additional ligands bind in a secondary coordination shell. Infrared resonance-enhanced photodissociation spectroscopy in the region of the CH4 a 1 and t 2 fundamental transitions reveals essentially free internal rotation of the core ligands about the H4C–Au+–CH4 axis, with sharp spectral features assigned by comparison with spectral simulations based on density functional theory. In separate experiments, vibrationally-enhanced dehydrogenation is observed when the t 2 vibrational normal mode in methane is excited prior to complexation. Clear infrared-induced enhancement is observed in the mass spectrum for peaks corresponding 4u below the mass of the Au+(CH4) n=2,3 complexes corresponding, presumably, to the loss of two H2 molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Isolated gas-phase metal ion–ligand clusters in many ways represent ideal model systems for studying fundamental metal–ligand interactions. Studies of these systems provide insight into metal–ligand binding energies and weakening of ligand internal bonds while remaining free from complex solvation, counterion, or defect effects. In turn, these shed light on the mechanism by which ligand activation occurs, leading to enhanced understanding of catalytic reactivity. Many industrial heterogeneous catalysts typically comprise multiple components, with reactions assumed to occur at metal defects supported by a macroscopic bulk structure [1]. The model systems studied can capture some of the features and energetics of the surface of catalysts [2], without complications arising from the bulk structure. The simplified nature of these model complexes also makes them tractable to theoretical studies which, coupled with experimental data, can provide a deeper understanding of fundamental interactions involved. These studies are often too computationally-intensive to perform rigorously on real systems.
The interaction and reaction of methane with transition metal ions is of particular practical interest. As the simplest saturated hydrocarbon, methane serves as a key model for understanding metal ion–hydrocarbon systems. The activation of methane is an intensively studied topic in catalysis [3,4,5]. Methane is the primary component in natural gas (typically 80–90%—[4]) and with depleting petroleum reserves, the possibility of easily converting methane to more valuable chemicals and fuels would lead to its use as an abundant hydrocarbon feedstock.
For complete methane activation to occur, at least one of the four strong C–H bonds (bond dissociation energy of 439 kJ mol−1—[6]) must undergo bond scission. This is made challenging by the paucity of low-energy empty and high-energy filled orbitals in methane, making it relatively inert to reaction under most conditions [3]. The classic industrial route used to convert methane into useful reagents involves the initial conversion of methane to syngas (CO + H2) via steam reforming, followed by the conversion of this syngas into a range of hydrocarbons or alcohols [3,4,5]. Despite representing a useful H2 source, the steam reforming of methane is energy-intensive, requiring high temperatures and consequent high capital costs associated with industrial operations [3, 4].
Many studies have investigated bulk-phase catalysts and reaction environments for directly converting methane into desired chemicals in high yields avoiding the syngas route, as reviewed by Tang et al. [3]. The ultimate goal is to better understand the fundamental interactions and mechanisms underlying the key catalytic processes in order to develop cost-efficient strategies for methane (and other hydrocarbons) activation. To this end, a wide variety of fundamental experimental and theoretical studies have investigated the reactivity of bare transition metal monocations with methane [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. These methods have been extensively reviewed and highlight the varied conditions and energetic requirements that yield particular products upon reaction [57,58,59,60,61,62].
The metal-catalysed dehydrogenation of methane (Reaction 1) is one reaction of key interest. This reaction is thermodynamically-feasible (i.e., exothermic) if the metal–methylidene bond dissociation energy exceeds the heat of dehydrogenation of methane (∆r H = 464 kJ mol−1 [35, 60, 61]). This reaction often begins with the formation of an M+(CH4) adduct followed by C–H bond insertion via oxidative addition to yield a hydridomethyl intermediate (H–M+–CH3). From here, H–H bond coupling and subsequent H2 elimination occurs via α-hydrogen transfer to yield a metal–methylidene product (MCH2 +) [40, 59, 61].
Of the ground-state transition metal ions investigated, 5d transition metal cations (specifically, Ta+, W+, Os+, Ir+, and Pt+) dehydrogenate methane the most efficiently [13, 15, 16, 35]. Ground-state 3d and 4d transition metals (with the notable exception of Nb+ [35]) rather than dehydrogenating methane, tend to undergo CH4 addition reactions leading to the formation of M+(CH4) n ion–molecule complexes [7, 35], often without substantial C–H bond activation. Unlike the ground state, electronically-excited Cr+ does dehydrogenate methane exothermically [55] as does ground-state Ti+ in coordination to multiple methane ligands [41]. In the latter case, complexation reduces the barrier to ion insertion into the C–H bond, thus favouring the formation of the key hydridomethyl intermediate in Ti+(CH4) n (n ≥ 3) complexes [41]. Extensive guided ion beam experiments have also shown that Reaction 1 occurs for various 3d and 4d transition metal monocations under single collision conditions at high collision energies. Modelling of the endothermic cross-sections can then provide metal–hydrogen and metal–carbon bond dissociation energies [8,9,10,11,12, 14, 18, 19, 23, 24, 26, 28, 29, 63].
Despite the extensive study of methane dehydrogenation, investigations focussing on the M+(CH4) adduct remain scarce. In this adduct, the bonding is believed to involve a combination of electrostatic and covalent interactions, with the hapticity controlled by the dominant interaction. For example, in Cu+(CH4), electrostatics favour η3 coordination whilst covalent interactions favour η2 coordination—[64]. The covalent interaction can generally be described with a simple donor–acceptor model [61] in which a σ-complex is formed involving the donation from C–H σ orbitals into (partially) empty orbitals on the metal, with concomitant back-donation from occupied metal π–orbitals into antibonding C–H orbitals. In some cases, this model has been expanded to include the effects of s–d hybridization, which often leads to a reduction of repulsion between the M+ and CH4 along the axis of interaction and an increased electron donation from proximate C–H σ bonds into vacant s–d hybridized orbitals [50, 56]. Overall, the M+–CH4 interaction leads to a red-shift in the stretching vibrations associated with proximate C–H bonds, the magnitude of which is sensitive to the electronic configuration (including the influence of promotion energies), orbital sizes, and exchange energy [16]. For M+(CH4) n complexes, the number and coordination environment of each ligand also influence the extent of C–H activation.
Overall, the red-shift in the stretching vibrations of the C–H bonds of CH4 upon metal complexation makes photofragmentation spectroscopy an ideal technique to probe the geometry of M+(CH4) n (n ≥ 1) complexes and hence the degree of C–H activation [65,66,67]. The s-block metal complexes Mg+(CH4) [68] and Ca+(CH4) [69], and transition metal complexes V+(CH4) [70] and Zn+(CH4) [71], have been studied via UV/Visible photofragmentation action spectroscopy in the vicinity of atomic metal ion resonances. Previous IR spectroscopic studies of M+(CH4) n complexes include those of s-block and p-block metals Li+(CH4)1−6 [72, 73] and Al+(CH4)1−6 [74], respectively. These complexes were found to be dominated by electrostatic interactions due to the inert electronic configurations of Li+ and Al+, with high charge density and a small ionic radius also contributing to the former. No measurable effects of ligand–ligand interactions in the first and second coordination shells were observed for these complexes. These electrostatic interactions were found to weaken as the number of ligands increases (characterised by a smaller red-shift), and as competition for interaction with the metal ion increases.
First-row transition metal complexes such as Mn+(CH4)1−6 [75], Fe+(CH4)1−4 [76], Co+(CH4)1−4 and Ni+(CH4)1−4 [77] have also been investigated via IR photofragmentation spectroscopy [75]. The Fe+, Co+ and Ni+ ions, with their 3d n ground state configurations, interact with methane more strongly than ions with 3d n−14s 1 configurations, such as Mn+(CH4)1−6. IR photofragmentation spectroscopy studies have also been performed on the d 10 complexes Cu+(CH4)1−6 and Ag+(CH4)1−6 [78], which tend to possess highly-symmetrical structures due to the spherical nature of the ion. The latter studies inspired the work presented here as part of our own development of a metal–ligand complex infrared dissociation instrument.
Despite all of the photofragmentation spectroscopy investigations on numerous M+(CH4) n systems performed previously, there remains a paucity of studies involving 5d transition metal ions. Of these, Au+ is of particular interest owing to its prolific use in homogenous catalysis [79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109], the emergence of which has been motivated by the unique properties of Au that are governed by strong relativistic effects [110,111,112,113,114,115]. Previous work on Au+(CH4) n complexes (n ≥ 1) includes an ICP/SIFT study involving the reactivity of Au+ with CH4 by Bohme and co-workers [35]. They observed a low propensity for formation of high-order Au+(CH4) n complexes with no dehydrogenation products observed. A combined guided ion beam/theoretical study by Li and Armentrout [33] observed a unique mechanism for the dehydrogenation of methane by Au+ which occurs without the involvement of an oxidative addition intermediate. Instead, AuCH2 + and H2 apparently form directly from the Au+(CH4) adduct without the involvement of a transition state, making this mechanism unique among the 5d transition metals.
Here, we report the results of an IR-REPD spectroscopic investigation of gas-phase Au+(CH4) n complexes (n = 3–8), employing the inert messenger or “rare-gas tagging” technique, whereby loss of a weakly-bound argon atom provides a mass spectrometric signature of IR photon absorption. This technique has previously been utilized by our group in the IR-REPD studies of M+(CO2) n complexes (M = Co, Rh, Ir) [116], M+(N2O) n complexes (M = Cu, Ag, Au) [117] and IR-MPD studies of larger bare and decorated transition metal clusters [118,119,120,121,122,123,124]. In separate experiments, we have observed enhanced dehydrogenation when methane is vibrationally excited prior to interaction with Au+.
2 Experimental and Computational Details
The instrument used in these studies has been described previously [116] and only basic details are given here. Gold ions are produced by pulsed laser ablation of a rotating gold disk target using 5 mJ of 532 nm light from a frequency doubled Nd:YAG laser (Continuum Minilite, 8 ns pulse). For rare-gas tagging, the ions are entrained in a pulse of He carrier gas, seeded with 2% methane and 20% argon, delivered by a solenoid valve (Parker-Hannifin, Series 9).
IR photodissociation of the argon-tagged complexes is performed using tunable IR light, generated from an OPO/OPA laser scanned in the region of the a 1 symmetric stretch (2917 cm−1) and t 2 stretch (3019 cm−1) of free CH4. Whenever the incident IR light is resonant with an IR-active mode of an Au+(CH4) n Ar complex, photons can be absorbed and, following intramolecular vibrational redistribution (IVR), the weakly-bound Ar atom is lost providing a spectral signature of the photon absorption. IR-REPD spectra are generated by monitoring the efficiency of Ar loss from the parent complex, as a function of wavelength.
To aid in the interpretation of the experimental spectra, we have performed spectral simulations of low-energy isomers using density functional theory. A range of candidate starting structures for Au+(CH4) n Ar and Au+(CH4) n were optimized using the B3P86 density functional [125, 126] coupled with the Def2TZVP basis set [127, 128]. Using the Def2TZVP basis set allows for 60 core electrons of Au to be treated with a quasirelativistic ab initio pseudopotential developed by Andrae et al. [129], which has been used successfully by our group to rationalize binding trends observed for M+(N2O) n complexes (where M = Cu, Ag, Au) [117]. From harmonic vibrational frequency calculations of the structures obtained, IR spectra were simulated by convoluting the line spectra with a Lorentzian line shape to match the experimental resolution. The simulated IR spectra were scaled for comparison with experimental IR-REPD spectra. The scaling factor (0.95538) was derived from comparison of the calculated 3160 cm−1 t 2 stretching frequency of CH4 with the experimental value of 3019 cm−1. Calculations were performed both with and without Ar atoms in order to determine any effects of the rare gas tag. All calculations were performed using the Gaussian09 suite of programs [130].
3 Results and Discussion
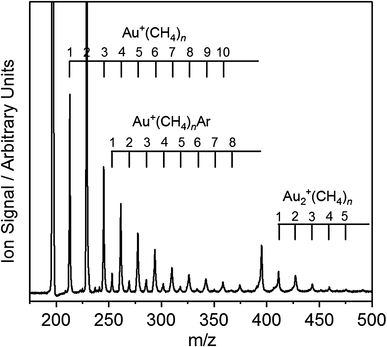
Figure 1 shows a typical time-of-flight mass spectrum obtained by laser ablation of a gold target in the presence of 2% CH4/20% Ar in He gas mixture at a backing pressure of 6 bar. The range of clusters generated can be tuned crudely with a combination of backing gas composition and backing pressure. A range of Au+(CH4) n , Au2 +(CH4) n and Au+(CH4) n Ar complexes can be assigned as shown. The Au+(CH4)2 complexes are produced with the strongest intensities—an early indication of the particular stability of this coordination number. In the absence of Ar tagging, and/or at higher CH4 partial pressures, the n = 2 complex is also the most intense for the Au2 + complexes.
Time-of-flight mass spectra of Au+(CH4) n , Au2 +(CH4) n , and Au+(CH4) n Ar complexes produced upon laser ablation of a gold target in the presence of a 2% CH4/20% Ar in He gas mixture at 6 bar backing pressure. The Au+(CH4)2 complex is produced with the highest signal intensity, reflecting the stability of this species
3.1 IR Depletion Spectra of Au+(CH4) n Ar Complexes
Under the experimental conditions employed here, Ar-tagging is successful for Au+(CH4) n up to n = 8 generating signals up to 10% of the corresponding non-tagged peaks. IR depletion spectra were obtained with acceptable signal-to-noise for the n = 3–8 complexes.
Figure 2 shows the IR depletion spectra recorded for the Au+(CH4) n Ar (n = 3–8) complexes in the region 2800–3200 cm−1 which encompasses the fundamental transitions of the t 2 and a 1 normal modes in free CH4. All spectra in this range are very similar each displaying three notable features: (i) A narrow band appearing at ca. 2900 cm−1, (ii) a broad, largely unresolved feature at ca. 3000 cm−1, and (iii) a weaker feature appearing at ca. 3065 cm−1.
The narrow intense band at ca. 2900 cm−1 can be assigned to a totally-symmetric a 1 C–H stretch in CH4 that becomes IR active upon complexation with Au+. This band is only slightly red-shifted relative to the a 1 fundamental in free CH4 (red-dashed line in Fig. 2, ca. 2917 cm−1 [131]). As the number of CH4 ligands (n) increases, this band blue-shifts slightly by ca. 3 cm−1 as the interaction of the Au+ with an increasing number of ligands diminishes the perturbation on individual CH4 moieties. The other features in the IR spectra are related to the methane t 2 stretch (whose wavenumber in free CH4, ca. 3019 cm−1, is marked by a blue-dashed line in Fig. 2 [132]).
Qualitatively similar features within the same IR range have also been observed in the IR depletion spectra of the other d 10 metal cation complexes, Cu+(CH4)4−6 and Ag+(CH4)5−6 recorded previously by Metz et al. [78]. However, for n = 3 the IR depletion spectra presented here for the gold complexes differ significantly from those of the other coinage metals indicating a markedly different binding motif.
3.2 DFT Calculations
3.2.1 Structural Dynamics and Band Analysis
For the n = 3 complex, DFT calculations yield two near-equivalent minima of significance as shown in Fig. 3. Both comprise a linear Au+(CH4)2 core structure in which the methane ligands are η 2 coordinated to the Au+ center, with a third, more weakly-bound ligand in a T-shaped structure. The primary difference between the two minima is whether the proximal hydrogens in the core ligands are staggered (Structure I—Fig. 3) or eclipsed (Structure II—Fig. 3) when viewed along the C–Au–C axis. These two structures are essentially isoenergetic with DFT predicting structure I to lie higher in energy by ca. 1 meV.
a Low-resolution IR-REPD spectrum of Au+(CH4)3Ar compared with simulated IR spectra of the: c eclipsed, and d staggered minima calculated at the UB3P86/Def2TZVP level of theory, with a scaling factor of 0.95538 applied to the simulated spectra. A simulated IR spectrum (b) generated by summing together individual IR spectra taken at various orientations between the two minima is also shown. Free internal rotation washes out the spectral features below 2850 cm−1 which arise from proximal C–H stretches in the core ligands. The dashed red lines indicate the positions of the peaks in the experimental IR spectrum. Calculations show that including the argon tag has minimal effect on the Au+(CH4)3 complex (and most likely Au+(CH4) n, generally)
Comparison of the IR-depletion spectrum for Au+(CH4)3Ar with the simulated IR spectra of both structures reveal interesting insights into the structural dynamics of this complex (Fig. 3c, d). The spectra of the two structures are nearly identical in the range 2850–3200 cm−1 with predicted features clearly recognizable in the experimental spectrum. However, strong additional bands—different for each structure—are observed in spectra simulated in the range 2400–2850 cm−1, where the experimental spectrum shows no such features. It is clear from the simulations that the wavenumbers of these lower-energy bands depend strongly upon the relative dihedral angle between the two core methane ligands. A relaxed scan of this dihedral angle between the eclipsed and staggered minima (Fig. 4) reveals a negligible 3 meV barrier to internal rotation, implying free rotation of the two inner-shell methane molecules. The effect of such motion is to wash out the spectral features in the 2400–2850 cm−1 region from the IR-REPD spectrum leaving only the persistent bands > 2850 cm−1 (see Fig. 3b) to be observed in the spectrum.
Further confirmation of the effects on the IR spectrum of free rotation of the core ligands is provided by the interpretation of the bands in the simulated IR spectrum of Structure I, shown in Fig. 5. The ten predicted bands in the IR spectrum can be grouped into five distinct groups based on the molecular motions involved.
The two bands at ca. 2550 cm−1 arise from combinations of symmetric proximal H–C–H stretches of the two core methane ligands. The second group—the two weaker bands at ca. 2750 cm−1—involves the anti-symmetric proximal H–C–H stretches of the same ligands. All of these bands are exquisitely sensitive to the dihedral angle and their wavenumber and intensity change continuously with the relative angle of the two ligands.
The rest of the bands observed in the IR-REPD spectrum of the n = 3 complex arise from motion either involving the outer ligand or distal C–H stretches of the core ligands. The 2900 cm−1 band can be confidently assigned due to a fully-symmetric (a 1) C–H stretch in the third, more weakly–bound ligand. The broad feature observed experimentally at ca. 3000 cm−1 has contributions from different t 2-type stretches including symmetric distal H–C–H stretches of the inner-core methane ligands, and three t 2 modes of the weakly-bound ligand (whose degeneracy is lifted by the interaction with Au+). Finally, the feature at 3065 cm−1 arises from the anti-symmetric distal H–C–H stretches (also of t 2 symmetry relative to free CH4) of the core ligands. These same three groups also appear in the simulated spectrum of the eclipsed structure (Structure II—Fig. 3), confirming their persistence during internal rotation of the core ligands.
The binding of the two core ligands in a η 2 fashion suggests that these interactions have significant covalent character. This is consistent with the calculations of Maitre and Bauschlicher on Cu+(CH4) [64], in which η 2 coordination to the Cu center arises due to covalent interactions whereas η 3 coordination, by contrast, arises from electrostatic interactions. Metz and co-workers [78] offer more specific details of the covalent interaction, concluding that η 2 coordination involves two charge transfer processes: (i) σ-donation from the Cu 3d z 2 orbital to empty anti-bonding ligand orbitals, and (ii) σ-back-donation from the CH4 t 2 bonding orbitals into the empty Cu 4s orbital. Equivalent interactions are likely to govern the interactions in the Au+(CH4)2 core.
In an attempt to simulate the likely effect on the spectrum of the free internal rotation, we have convoluted the spectra calculated for structures at every 5° of dihedral angle between 0 and 90 (see Fig. 3 b). As expected, only the spectral features arising from the distal core ligands and those associated with the outer coordination shell ligands persist with significant intensity and the spectrum provides a satisfactory fit to the experimental data.
For the rest of the Au+(CH4) n Ar complexes (n = 4–8) there is good agreement between the experimental IR-REPD spectra and the simulated spectra of their putative global minimum structures. This is to be expected as each calculated structure comprises the same linear Au+(CH4)2 core (with mean Au+–CH4 bond lengths of ca. 2.3 Å) to which additional methane ligands bind equatorially up to n = 6 (with average Au+–CH4 bond lengths of ca. 3.5 Å) until a second coordination shell is complete. Beyond n = 6, additional methane molecules bind at even larger Au+–CH4 bond lengths (ca. 5.8 Å).
Since the IR spectrum is dominated by features in the common core only minor changes are observed with increasing ligand number. The assignments made for the n = 3 complex apply to all complexes, with visualization of the numerous modes for all global minima confirming this assertion. The slight blue-shift and broadening of the experimental band at ca. 2900 cm−1 as n increases is due to an increasing number of outer ligands contributing to this particular feature. Likewise, the increasing number of outer ligands accounts for the increase in relative intensity of the broad 3000 cm−1 feature.
3.2.2 Trends in Binding Energy and Au+–C Bond Distances
Further evidence for an inner-core of two methane ligands in Au+(CH4) n Ar complexes is provided by both: (i) the DFT-calculated binding energy of CH4 ligands, and (ii) the longest Au+–C internuclear separation in each complex as a function of ligand number (see Fig. 6). The first two ligands to bind do so with large binding energies (ca. 1.2 eV). This is a factor two larger than the equivalent binding energies calculated by Kocak et al. for Ag+(CH4)1,2 and larger even than for Cu+(CH4)1,2 [78]. By contrast, the third ligand has a binding energy more than an order of magnitude smaller.
The greatest Au–C internuclear separation (red, right hand scale) and binding energy of the nth methane (blue, left hand scale) in Au+(CH4) n (n = 1–8) plotted against the number of methane ligands, n. The step-changes between n = 2 and 3, and between n = 6 and 7 indicate the existence of a core structure with two methane ligands and a second-shell of four methane ligands
The same effect is mirrored in the trends in Au+–C internuclear separation. The inner, core ligands have mean metal–ligand separations of 2.3 Å but the third ligand binds at a mean distance of 3.5 Å away. A second step in this trend is observed between n = 6 and 7 as the second solvation shell closes, the 7th ligand is a distant 5.8 Å from the metal centre.
The observation of a linear Au+(CH4)2 core structure in the complexes studied here is not surprising—Au+–L2 structures are common. As first postulated by Orgel [133], this behavior is often attributed to hybridization of the 6s and 5d z 2 orbitals of Au+—which is very efficient due to relativistic effects. These s–d z hybrid orbitals favor the linear interaction of two ligands on opposing sides of the Au+ center.
3.3 Reactions of Au+/Au+(CH4) n with Vibrationally-Excited CH4
The IR-REPD experiments above rely on first forming metal–ligand complexes and then interrogating them downstream in the molecular beam with the IR pulse. We have also performed a conceptually different series of experiments in which the IR pulse is fired just before the metal ablation laser. This is made possible by the counter-propagating nature of the IR laser and the molecular beam in our instrument which allows the IR laser to penetrate through the skimmer orifice right up to the cluster source.
In these experiments only the IR active, t 2 stretching mode of free methane at 3019 cm−1 is excited. As shown in Fig. 7, vibrationally-exciting the methane in the beam has two significant effects: (i) it results in a reduction in the generation of Au+(CH4) n complexes (particularly n > 2), and (ii) it leads to an enhancement in minor peaks in the mass spectrum 4u lower than the main Au+(CH4) n complexes’ peaks.
a Mass spectra of Au+(CH4) n complexes without (black) and with (red) the IR laser pulse, resonant on the Q-branch in the CH4 t 2 fundamental band at 3019 cm−1, fired immediately before the ablation laser pulse. The asterisks indicate observed enhancement in signals at m/z = 225 and 241 assigned to dehydrogenation products arising from reactions with vibrationally excited CH4. b The corresponding IR spectrum of the depletion signal on Au+(CH4)3 (blue) indicating reduced complexation efficiency upon ligand IR excitation and enhancement (red) of the Au+[C3, H8] signal at m/z = 241
The reduction in the efficiency of complexation following vibrational excitation of the ligand is unsurprising—this represents additional internal energy which must be lost in subsequent cooling collisions for the complex to remain bound. Furthermore, IR excitation of CH4 in the comparatively high pressure region of the cluster source most likely produces a photoacoustic signal and shock-wave which heats the beam locally. When the IR laser is resonant with the Q-branch of the t 2 fundamental, the signal of the n = 3 complex is reduced by 80% and is accompanied by almost complete loss of the larger complexes (see Fig. 7a).
The reduction in the efficiency of generating Au+(CH4) n complexes resulting from CH4 vibrational excitation is sufficiently strong to permit a spectrum of this “complex inhibition” to be recorded (Fig. 7b), recorded in depletion of the Au+(CH4)3 signal as a function of IR wavenumber. This spectrum reflects well the IR absorption spectrum of CH4 of the t 2 fundamental band with preliminary modelling of the spectral band contours suggesting a slight cooling within the short cluster channel following expansion from the valve.
The second, more interesting, effect observed in Fig. 7 is a marked enhancement in the intensity of peaks in the mass spectrum corresponding to dehydrogenation products following reaction with vibrationally-excited methane molecules. This is particularly noticeable at m/z = 225 (corresponding to Au+(CH4)2–4u) and 241 (Au+(CH4)3–4u), respectively). In the same way that the CH4 IR absorption spectrum is reproduced in the depletion of Au+(CH4) n , the same spectrum is manifest in the absolute enhancement of the signal observed at m/z = 241 (see Fig. 7b), confirming the role of the infrared excitation of CH4 in the production of this species.
Clearly these absolute enhancements imply an increase in reactivity as a result of IR excitation of the CH4 though we can only speculate as to: (i) the reaction(s) which is(are) enhanced (CH4* + Au+ or CH4* + Au+(CH4) n ), and (ii) the nature of the reaction products since we have no explicit spectral information beyond the mass of the species produced. In this context, it is worth noting that, in their recent mid-IRMPD study of the results of Pt+ + CH4 reactions/clustering, Bakker, Armentrout and co-workers observed significant production of Pt+(CH3)2(CH4) n from collisions during the clustering processes [134]. This product was interpreted as arising from methane C–H activation by Pt+ generating Pt+CH2 which subsequently reacts with additional methane to produce the di-methyl complexes. In a wider context, Beck and coworkers have studied vibrationally-mediated dissociative chemisorption of CH4 isotopologues on extended transition-metal surfaces observing mode-specific dissociation [135,136,137].
More generally, single dehydrogenation of methane (i.e., H2 loss) within M+(CH4) n complexes (M = Nb and Ta; n = 1–4) has been investigated theoretically previously, and two common reaction mechanisms—termed ‘direct activation’ and ‘cluster-assisted activation’—are believed to form two different products [47]. The ‘direct activation’ mechanism involves oxidative insertion of M+ into a C–H bond followed by H migration leading to formation of the (H2)–M+–(CH2) intermediate, followed by reductive elimination of H2 (Scheme 1):
Under single collision conditions, Irikura and Beauchamp found several third-row transition metal ions, namely W+, Ta+, Os+, Ir+ and Pt+ to dehydrogenate methane consistent with this mechanism. Au+, however, was found to be unreactive with methane under ion cyclotron resonance conditions but did slowly dehydrogenate ethane [15, 16].
More recently, in guided ion beam studies, Li and Armentrout found that, in the case of Au+ + CH4, due to the 5d 10 ion configuration the barrier for the oxidative addition/reductive elimination reaction is much too high to account for the reaction efficiencies they observed at low collision energies. Instead dehydrogenation occurs, endothermically, directly from the Au+(CH4) adduct without an intermediate transition state [33].
Within n > 2 complexes, in addition to the mechanism outlined in Scheme 1 which generates species of the form M+(CH2)(CH4) m , a ‘cluster-assisted’ mechanism leading to the formation of another product is also plausible whereby insertion into a C–H bond is followed by H migration from a different methane ligand followed by H2 loss to leave the dimethyl complex (Scheme 2):
In our study, only double-dehydrogenation (i.e., loss of two units of H2) is observed clearly, resulting in enhancements of the Au+[C2, H4] and Au+[C3, H8] species. We cannot exclude the possibility of some H2 loss with our mass resolution, but there is no obvious peak in Fig. 7 at m/z 243. Nevertheless, it is possible to conceive extended versions of the above which result in the loss of two H2 molecules. Clearly, reaction pathway calculations are required which would be informed by spectroscopy of the reaction products. This would require post-reaction spectroscopic studies of the type performed by Wheeler et al. [134] and the lack of availability of multiple IR lasers prevented such studies here.
4 Conclusions
Infrared spectroscopy combined with spectral simulations based on density functional theory have revealed a linear core Au+(CH4)2 structure to small gas-phase Au+(CH4) n metal–ligand complexes. The core ligands bind in a η 2 motif with evidence of incipient chemical bonding with additional ligands binding closer to η 3. In the region of the CH4 a 1 and t 2 vibrational modes, free internal rotation of the core washes out several spectral features leaving only persistent bands based on distal CH2 stretches and vibrations in non-core ligands.
Vibrational excitation of methane before interactions with metal atoms/ions are possible, leads to an expected reduction in the number density of complexes produced but also an enhancement in (double–) dehydrogenation products. This latter observation, arising presumably from enhanced C–H activation in the vibrationally-excited ligand, offers the possibility of mode-selective intra-cluster chemistry similar to that observed in dissociative chemisorption of methane on extended transition metal surfaces.
References
Harding DJ, Fielicke A (2014) Chem-Eur J 20(12):3258–3267
Lang SM, Bernhardt TM (2012) Phys Chem Chem Phys 14(26):9255–9269
Tang P, Zhu QJ, Wu ZX, Ma D (2014) Energy Environ Sci 7(8):2580–2591
Crabtree RH (1995) Chem Rev 95(4):987–1007
Schwach P, Pan XL, Bao XH (2017) Chem Rev 117(13):8497–8520
Blanksby SJ, Ellison GB (2003) Accounts Chem Res 36(4):255–263
Tonkyn R, Ronan M, Weisshaar JC (1988) J Phys Chem 92(1):92–102
Armentrout PB, Beauchamp JL (1981) J Am Chem Soc 103(4):784–791
Aristov N, Armentrout PB (1987) J Phys Chem 91(24):6178–6188
Schultz RH, Elkind JL, Armentrout PB (1988) J Am Chem Soc 110(2):411–423
Sunderlin LS, Armentrout PB (1988) J Phys Chem 92(5):1209–1219
Georgiadis R, Armentrout PB (1988) J Phys Chem 92(25):7067–7074
Buckner SW, Macmahon TJ, Byrd GD, Freiser BS (1989) Inorg Chem 28(18):3511–3518
Sunderlin LS, Armentrout PB (1989) J Am Chem Soc 111(11):3845–3855
Irikura KK, Beauchamp JL (1991) J Am Chem Soc 113(7):2769–2770
Irikura KK, Beauchamp JL (1991) J Phys Chem 95(21):8344–8351
Schultz RH, Armentrout PB (1993) J Phys Chem 97(3):596–603
Chen YM, Armentrout PB (1995) J Phys Chem 99(27):10775–10779
Haynes CL, Chen YM, Armentrout PB (1995) J Phys Chem 99(22):9110–9117
Haynes CL, Armentrout PB, Perry JK, Goddard WA (1995) J Phys Chem 99(17):6340–6346
Heinemann C, Wesendrup R, Schwarz H (1995) Chem Phys Lett 239(1–3):75–83
Haynes CL, Chen YM, Armentrout PB (1996) J Phys Chem 100(1):111–119
Chen YM, Sievers MR, Armentrout PB (1997) Int J Mass Spectrom Ion Process 167:195–212
Sievers MR, Chen YM, Haynes CL, Armentrout PB (2000) Int J Mass Spectrom 195:149–170
Zhang XG, Liyanage R, Armentrout PB (2001) J Am Chem Soc 123(23):5563–5575
Armentrout PB, Sievers MR (2003) J Phys Chem A 107(22):4396–4406
Armentrout MM, Li FX, Armentrout PB (2004) J Phys Chem A 108(45):9660–9672
Liu F, Zhang XG, Armentrout PB (2005) Phys Chem Chem Phys 7(5):1054–1064
Armentrout PB (2006) J Phys Chem A 110(27):8327–8338
Armentrout PB, Shin S, Liyanage R (2006) J Phys Chem A 110(4):1242–1260
Li FX, Zhang XG, Armentrout PB (2006) Int J Mass Spectrom 255:279–300
Parke LG, Hinton CS, Armentrout PB (2006) Int J Mass Spectrom 254(3):168–182
Li FX, Armentrout PB (2006) J Chem Phys 125(13):133114
Simon A, MacAleese L, Boissel P, Maitre P (2002) Int J Mass Spectrom 219(3):457–473
Shayesteh A, Lavrov VV, Koyanagi GK, Bohme DK (2009) J Phys Chem A 113(19):5602–5611
Blomberg MRA, Siegbahn PEM, Svensson M (1994) J Phys Chem 98(8):2062–2071
Westerberg J, Blomberg MRA (1998) J Phys Chem A 102(37):7303–7307
Perry JK, Ohanessian G, Goddard WA (1993) J Phys Chem 97(20):5238–5245
Kemper PR, Bushnell J, Vankoppen P, Bowers MT (1993) J Phys Chem 97(9):1810–1817
Perry JK, Ohanessian G, Goddard WA (1994) Organometallics 13(5):1870–1877
van Koppen PAM, Perry JK, Kemper PR, Bushnell JE, Bowers MT (1999) Int J Mass Spectrom 185:989–1001
Russo N, Sicilia E (2001) J Am Chem Soc 123(11):2588–2596
Sicilia E, Russo N (2002) J Am Chem Soc 124(7):1471–1480
Michelini MD, Russo N, Sicilia E (2002) J Phys Chem A 106(38):8937–8944
Michelini MD, Sicilia E, Russo N, Alikhani ME, Silvi B (2003) J Phys Chem A 107(24):4862–4868
Chiodo S, Kondakova O, Michelini MD, Russo N, Sicilia E, Irigoras A, Ugalde JM (2004) J Phys Chem A 108(6):1069–1081
Sicilia E, Mazzone G, Perez-Gonzalez A, Pirillo J, Galano A, Heine T, Russo N (2017) Phys Chem Chem Phys 19(24):16178–16188
Sandig N, Koch W (1997) Organometallics 16(24):5244–5251
Ye S, Shi NH, Huang JH, Dai SS (1997) Int J Quantum Chem 62(1):23–27
Zhang Q, Kemper PR, Shin SK, Bowers MT (2001) Int J Mass Spectrom 204(1–3):281–294
Wang CJ, Xu X, Cao ZJ, Ye S, Zhang Q (2003) J Phys Chem A 107(34):6681–6687
Zhang GB, Li SH, Jiang YS (2003) Organometallics 22(19):3820–3830
Zhang DJ, Liu CB, Bi SW, Yuan SL (2003) Chem-Eur J 9(2):484–501
Premkumar JR, Sastry GN (2014) J Phys Chem A 118(48):11388–11398
Halle LF, Armentrout PB, Beauchamp JL (1981) J Am Chem Soc 103(4):962–963
Zhang Q, Kemper PR, Bowers MT (2001) Int J Mass Spectrom 210(1–3):265–281
Armentrout PB, Beauchamp JL (1989) Accounts Chem Res 22(9):315–321
Eller K, Schwarz H (1991) Chem Rev 91(6):1121–1177
Roithova J, Schroder D (2010) Chem Rev 110(2):1170–1211
Schwarz H (2011) Angew Chem Int Ed 50(43):10096–10115
Schwarz H (2014) Isr J Chem 54(10):1413–1431
Armentrout PB (2017) Chem-Eur J 23(1):10–18
Armentrout PB, Chen YM (2017) Int J Mass Spectrom 413:135–149
Maitre P, Bauschlicher CW (1993) J Phys Chem 97(46):11912–11920
Lisy JM (1997) Int Rev Phys Chem 16(3):267–289
Bieske EJ, Dopfer O (2000) Chem Rev 100(11):3963–3998
Duncan MA (2003) Int Rev Phys Chem 22(2):407–435
Cheng YC, Chen J, Ding LN, Wong TH, Kleiber PD, Liu DK (1996) J Chem Phys 104(17):6452–6459
Chen J, Cheng YC, Kleiber PD (1997) J Chem Phys 106(10):3884–3890
Hayes T, Bellert D, Buthelezi T, Brucat PJ (1997) Chem Phys Lett 264(1–2):220–224
Lu WY, Wong TH, Kleiber PD (2001) Chem Phys Lett 347(1–3):183–188
Rodriguez O, Lisy JM (2011) Chem Phys Lett 502(4–6):145–149
Rodriguez O, Lisy JM (2011) J Phys Chem A 115(7):1228–1233
Poad BLJ, Thompson CD, Bieske EJ (2008) Chem Phys 346(1–3):176–181
Dryza V, Bieske EJ (2010) Int J Mass Spectrom 297(1–3):46–54
Citir M, Altinay G, Austein-Miller G, Metz RB (2010) J Phys Chem A 114(42):11322–11329
Kocak A, Sallese Z, Johnston MD, Metz RB (2014) J Phys Chem A 118(18):3253–3265
Kocak A, Ashraf MA, Metz RB (2015) J Phys Chem A 119(37):9653–9665
Li ZG, Brouwer C, He C (2008) Chem Rev 108(8):3239–3265
Muzart J (2008) Tetrahedron 64(25):5815–5849
Jimenez-Nunez E, Echavarren AM (2008) Chem Rev 108(8):3326–3350
Widenhoefer RA (2008) Chem-Eur J 14(18):5382–5391
Arcadi A (2008) Chem Rev 108(8):3266–3325
Hashmi ASK, Rudolph M (2008) Chem Soc Rev 37(9):1766–1775
Della Pina C, Falletta E, Prati L, Rossi M (2008) Chem Soc Rev 37(9):2077–2095
Gorin DJ, Sherry BD, Toste FD (2008) Chem Rev 108(8):3351–3378
Garcia P, Malacria M, Aubert C, Gandon V, Fensterbank L (2010) ChemCatChem 2(5):493–497
Sengupta S, Shi XD (2010) ChemCatChem 2(6):609–619
Hopkinson MN, Gee AD, Gouverneur V (2011) Chem-Eur J 17(30):8248–8262
Corma A, Leyva-Perez A, Sabater MJ (2011) Chem Rev 111(3):1657–1712
Brenzovich WE (2012) Angew Chem Int Ed 51(36):8933–8935
Lu BL, Dai LZ, Shi M (2012) Chem Soc Rev 41(8):3318–3339
Brand JP, Li YF, Waser J (2013) Isr J Chem 53(11–12):901–910
Cera G, Bandini M (2013) Isr J Chem 53(11–12):848–855
Zuccaccia D, Belpassi L, Macchioni A, Tarantelli F (2013) Eur J Inorg Chem 2013(24):4121–4135
Joost M, Amgoune A, Bourissou D (2015) Angew Chem Int Ed 54(50):15022–15045
Joost M, Estevez L, Miqueu K, Amgoune A, Bourissou D (2015) Angew Chem Int Ed 54(17):5236–5240
Debrouwer W, Heugebaert TSA, Roman BI, Stevens CV (2015) Adv Synth Catal 357(14–15):2975–3006
Dorel R, Echavarren AM (2015) Chem Rev 115(17):9028–9072
Jia MQ, Bandini M (2015) ACS Catal 5(3):1638–1652
Asiri AM, Hashmi ASK (2016) Chem Soc Rev 45(16):4471–4503
Blanc A, Beneteau V, Weibel JM, Pale P (2016) Org Biomol Chem 14(39):9184–9205
Kumari ALS, Reddy AS, Swamy KCK (2016) Org Biomol Chem 14(28):6651–6671
Pflasterer D, Hashmi ASK (2016) Chem Soc Rev 45(5):1331–1367
Zi WW, Toste FD (2016) Chem Soc Rev 45(16):4567–4589
Holmsen MSM, Nova A, Balcells D, Langseth E, Oien-Odegaard S, Traseth EA, Heyn RH, Tilset M (2016) Dalton Trans 45(37):14719–14724
Liu L, Zhang JL (2016) Chem Soc Rev 45(3):506–516
Li YY, Li WB, Zhang JL (2017) Chem-Eur J 23(3):467–512
Shahzad SA, Sajid MA, Khan ZA, Canseco-Gonzalez D (2017) Synth Commun 47(8):735–755
Pyykko P (1988) Chem Rev 88(3):563–594
Schwerdtfeger P (2002) Heteroatom Chem 13(6):578–584
Pyykko P (2004) Angew Chem Int Ed 43(34):4412–4456
Pyykko P (2005) Inorg Chim Acta 358(14):4113–4130
Gorin DJ, Toste FD (2007) Nature 446(7134):395–403
Pyykko P (2008) Chem Soc Rev 37(9):1967–1997
Iskra A, Gentleman AS, Kartouzian A, Kent MJ, Sharp AP, Mackenzie SR (2017) J Phys Chem A 121(1):133–140
Cunningham EM, Gentleman AS, Beardsmore PW, Iskra A, Mackenzie SR (2017) J Phys Chem A. doi:10.1021/acs.jpca.7b07628
Hamilton SM, Hopkins WS, Harding DJ, Walsh TR, Gruene P, Haertelt M, Fielicke A, Meijer G, Mackenzie SR (2010) J Am Chem Soc 132(5):1448
Harding DJ, Walsh TR, Hamilton SM, Hopkins WS, Mackenzie SR, Gruene P, Haertelt M, Meijer G, Fielicke A (2010) J Chem Phys 132(1):011101. doi:10.1063/1.3285266
Harding DJ, Gruene P, Haertelt M, Meijer G, Fielicke A, Hamilton SM, Hopkins WS, Mackenzie SR, Neville SP, Walsh TR (2010) J Chem Phys 133(21):214304
Hamilton SM, Hopkins WS, Harding DJ, Walsh TR, Haertelt M, Kerpal C, Gruene P, Meijer G, Fielicke A, Mackenzie SR (2011) J Phys Chem A 115(12):2489–2497
Hermes AC, Hamilton SM, Hopkins WS, Harding DJ, Kerpal C, Meijer G, Fielicke A, Mackenzie SR (2011) J Phys Chem Lett 2(24):3053–3057
Hermes AC, Hamilton SM, Cooper GA, Kerpal C, Harding DJ, Meijer G, Fielicke A, Mackenzie SR (2012) Faraday Discuss 157:213–225
Kerpal C, Harding DJ, Hermes AC, Meijer G, Mackenzie SR, Fielicke A (2013) J Phys Chem A 117(6):1233–1239
Becke AD (1993) J Chem Phys 98(7):5648–5652
Perdew JP (1986) Phys Rev B 33(12):8822–8824
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7(18):3297–3305
Weigend F (2006) Phys Chem Chem Phys 8(9):1057–1065
Andrae D, Haussermann U, Dolg M, Stoll H, Preuss H (1990) Theor Chim Acta 77(2):123–141
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich A, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JJA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 09, Revision D.01. Gaussian Inc., Wallingford
Shimanouchi T (1972) Tables of molecular vibrational frequencies, vol consolidated volume 1 NSRDS-NBS 39. National Bureau of Standards, Washington
Person WB, Zerbi G (1982) Vibrational intensities in infrared and Raman spectroscopy. Elsevier, Amsterdam
Orgel LE (1958) J Chem Soc 4186–4190
Wheeler OW, Salem M, Gao A, Bakker JM, Armentrout PB (2016) J Phys Chem A 120(31):6216–6227
Bisson R, Sacchi M, Beck RD (2010) Phys Rev B 82(12):121404
Chadwick H, Beck RD (2017) Annu Rev Phys Chem 68:39–61
Chen L, Ueta H, Bisson R, Beck RD (2012) Faraday Discuss 157:285–295
Acknowledgements
EMC and AI are grateful to the EPSRC for graduate studentships. AEG is grateful to Magdalen College, Oxford for her Leon E and Iris L Graduate Scholarship. AI is also grateful to Wadham College, Oxford, for additional support. This work is supported by EPSRC under Programme Grant No. EP/L005913 and by the SCG Chemicals Co. via the Oxford Centre of Excellence Innovation Fund. We are grateful to Advanced Research Computing (ARC) facility at the University of Oxford for providing supercomputer facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gentleman, A.S., Green, A.E., Price, D.R. et al. Infrared Spectroscopy of Au+(CH4) n Complexes and Vibrationally-Enhanced C–H Activation Reactions. Top Catal 61, 81–91 (2018). https://doi.org/10.1007/s11244-017-0868-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0868-z