Abstract
Au/Pd nanoparticles are important in a number of catalytic processes. Here we investigate the formation of Au–Pd bimetallic nanoparticles on TiO2(110) and their susceptibility to encapsulation using scanning tunneling microscopy, as well as Auger spectroscopy and low energy electron diffraction. Sequentially depositing 5 MLE Pd and 1 MLE Au at 298 K followed by annealing to 573 K results in a bimetallic core and Pd shell, with TiOx encapsulation on annealing to ~ 800 K. Further deposition of Au on the pinwheel type TiOx layer results in a template-assisted nucleation of Au nanoclusters, while on the zigzag type TiOx layer no preferential adsorption site of Au was observed. Increasing the Au:Pd ratio to 3 MLE Pd and 2 MLE Au results in nanoparticles that are enriched in Au at their surface, which exhibit a strong resistance towards encapsulation. Hence the degree of encapsulation of the nanoparticles during sintering can be controlled by tuning the Au:Pd ratio.
Similar content being viewed by others
1 Introduction
Atomically dispersed bimetallic surface alloys are excellent nanocomposite materials for fine-tuning the active centers of a number of homogeneous [1], heterogeneous [2,3,4,5,6,7], photo-[8, 9] and electro-catalysts [6, 10, 11]. By systematically studying appropriate model systems, it is possible to establish the connection between their structure and activity [4, 12,13,14,15,16]. The exceptional and enhanced catalytic properties of alloyed, bimetallic systems arises from the synergistic properties of the two metals. Indeed, the relationship between the surface free energies and work functions of the metals and their support plays a crucial role in surface and subsurface processes [17]. Furthermore, the activity and selectivity of these catalysts is influenced by the composition and morphology of the nanoparticles (NPs) [18, 19].
Pd–Au alloyed catalysts have proved to be excellent catalysts for a number of chemical processes such as the acetoxylation of ethylene to vinyl acetate [12, 20], solvent-free oxidation of primary alcohols to aldehydes [21] and direct synthesis of H2O2 from H2 and O2 [22]. Recently, it has been demonstrated that Au-core Pd-shell NPs in microbial fuel cells exhibit enhanced catalytic activity in wastewater treatment technology [23].
The formation of bimetallic NPs on reducible oxide surfaces is a rather complex process in that reduced species (such as O vacancies) on the oxide support play an influential role in the process of nanoparticle nucleation [24, 25]. The structure of the supported composite nanoparticles are largely determined by the nanoscale thermodynamics of their components and interfaces, which may lead to a large variation in their composition [26,27,28,29,30,31,32,33,34]. This tunability of composition offers considerable potential for enhanced selectivity and activity in catalytic applications [35, 36]. Encapsulation of supported metal NPs as a result of strong metal supported interaction (SMSI) can be expected on easily reducible oxide supports such as TiO2, TaO5, CeO2 and NbO for VIII.B metals (Ir, Rh, Ni, Pd and Pt) whose work function is above 5.3 eV and with a surface energy over 2 Jm− 2 [17].
In general, the encapsulation of the supported metal nanoparticles degrades their catalytic activity by decreasing the number of the catalytically active sites [37, 38], although SMSI enhancement of activity has also been reported [39]. Although it is possible to decorate a Au(111) surface with TiOx by oxidising a Ti adlayer [40], it does not appear possible to encapsulate Au nanoparticles on a reducible support. Moreover, a Au overlayer on a VIII. B metal can strongly hinder the SMSI process [31, 32], while alloy formation at higher temperatures can also occur [41, 42].
Associated with interest in graphene like 2D materials [43], there have been a number of studies of 2D oxide nanomaterials [31, 44, 45]. The commonly accepted stacking sequence of TiOx ultrathin oxide layers on transition metals is the following: M–Ti–Ox, where M corresponds to the transition metal bonded to the oxide substrate [46, 47]. The lattice mismatch and the rotation between the transition metal’s lattice and Ti lattice creates a moiré pattern with unit cell dimensions around 1.5 nm in the case of the pinwheel structure. This periodicity coincides with the superlattice’s unit cell’s corners, creating periodically lower-surface-potential areas (break in the homogeneous surface potential) in the oxide layer, which can act as a trapping site for adsorbed metal atoms. In connection with this property, template assisted adsorption and growth of Au nanoparticles has been investigated previously in the cases of Rh(111)/TiOx/Au [47] and Pt(111)/TiOx/Au [44]. In contrast, the adsorption properties of Au on a more complex system such as encapsulated bimetallic nanoparticles have not yet been reported.
Here we report the study of the influence of the Au:Pd ratio on the core–shell composition of nanoparticles on TiO2(110). Furthermore, we study the morphology of submonolayer Au deposited on the TiOx ultrathin oxide films formed on the supported Au–Pd nanoparticles.
2 Experimental
The experiments were carried out in London using an Omicron GmbH variable temperature scanning tunneling microscope (STM) housed in an ultrahigh vacuum chamber (base pressure = 5 × 10− 11 mbar). The system was also equipped with low energy electron diffraction (LEED) optics and a retarding field Auger-electron spectrometer (AES). TiO2(110) single crystals (Pi-Kem) were prepared by cycles of argon ion sputtering (1 kV) and annealing in vacuum at 1000 K. The sample cleanliness and long range order were confirmed using AES and LEED, respectively. Pd and Au were deposited onto the as-prepared TiO2(110) surface at room temperature. Dosing rates were calibrated with AES. The Pd (Au) evaporation source consisted of a Pd (Au) wire wrapped on tungsten filament that was resistively heated. For the formation of the bimetallic nanoparticles, the same deposition conditions were maintained such that the coverage of Au and Pd on TiO2(110) could be estimated from the volume of the nanoparticles measured with STM. The coverage of Pd and Au is expressed in equivalent monolayers (MLE), defined as the surface concentration of Pd(111) and Au(111), respectively (for Pd 1 MLE ~ 1.53 × 1015 atoms/cm2, while for Au 1 MLE ~ 1.39 × 1015 atoms/cm2). STM images were recorded in constant current mode using W tips prepared by electrochemical etching and conditioned by outgassing at 500 K as well as voltage pulses in STM. The STM images were analyzed using WSxM software [48]. For determining the average height, at least one hundred nanoparticles were measured.
3 Results and Discussion
3.1 Formation of a Pd Film (8 MLE) at RT on TiO2(110)-(1 × 1) and the effects of the annealing up to 1000 K
Figure 1a shows a typical STM image of the as-prepared TiO2(110)-(1 × 1) surface. The surface comprises large (1 × 1) terraces separated by step edges that run along the [001] and [1–11] crystallographic directions [49]. On the terrace (Fig. 1b), alternating bright and dark rows, corresponding to the rows of fivefold coordinated Ti (Ti5c) and twofold coordinated bridging O (Ob) ions respectively, run along the [001] direction. Bright features that link the neighboring Ti5c rows are the missing Ob ions, namely Ob vacancies (or Ob-vacs) [50, 51].
a, b STM images of as-prepared TiO2(110)-(1 × 1). c As a, after adsorption of 8 MLE Pd, followed by annealing at 973 K for 1 h. d Atomically resolved images from the Pd nanoparticle top facets that exhibit pinwheel (upper) and zig–zag (lower) encapsulation structures. Image sizes: a 30 × 30, b 6 × 6, c, d 6 × 3 nm2. e LEED pattern (E = 48 eV) taken from the surface in c. f Assignments of the LEED pattern. The red rectangle marks the unit cell of TiO2(110)-(1 × 1), while the black hexagon marks the unit cell of Pd(111), revealing a calculated lattice constant of 2.7 ± 0.15 Å. The orange hexagon marks the pinwheel structure, while the yellow, green and blue hexagons mark pinwheel-like structures. Scan parameters: a–c VS, IT = + 1.5 V, 1.0 nA and d + 0.2 V, 5 nA
Deposition of eight monolayer equivalents (MLE) Pd onto TiO2(110) results in the formation of a continuous multilayer film of Pd that dewets partially after annealing at 953 K for 1 h (Fig. 1c). The morphology of the film largely follows that of the substrate, with some quasi-hexagonal holes that develop inside the film. Atomically resolved STM images recorded from the top part of the Pd film (Fig. 1d) reveal that it is encapsulated by the well-known pinwheel and zigzag TiOx structures as a result of strong metal–support interaction (SMSI) [49, 52, 53]. The pinwheel structure has a hexagonal unit cell with cell length of 1.7 ± 0.1 nm (black lines in the top part of Fig. 1d), oriented 30° with respect to the [001] direction of the TiO2(110) substrate, while a dotted white circle picks out the pinwheel structure. The measured interatomic distance in the pinwheel structure is 3.3 ± 0.1 Å. The zig–zag TiOx structure has a rhombic unit cell with cell parameters of 0.8 × 0.66 nm2 (black lines, bottom part of Fig. 1d). The interatomic distances in the zigzag structure are 2.9 ± 0.1 and 3.3 ± 0.1 Å. The trough and the glide plane of the zigzag is marked with white dotted lines, while the continuous white lines depict the zigzag structure in the bottom part of Fig. 1d. These results are in a good agreement with earlier work [54, 55].
We also performed LEED on the same surface. As shown in Fig. 1e, f, the LEED pattern is composed of diffraction spots of the TiO2(110) substrate (red squares in Fig. 1f) and those of the (111) top-facet of the Pd multilayers (black hexagons), from which the nearest neighbour distance of Pd atoms of the Pd(111) top-facet was calculated to be 2.70 ± 0.15 Å. In addition, the LEED pattern also has diffraction spots that originate from different TiOx encapsulation layers. These include the diffraction spots originating from the pinwheel structure (orange hexagons, rotated by ± 3° with respective to the Pd(111) pattern), as well as the diffraction patterns that we attribute to other pinwheel-like structures (blue, green, and yellow respectively in Fig. 1f). The unit-cell parameters of the pinwheel as well as of pinwheel-like structures are listed in Table 1. Although we observed the zigzag TiOx structure in STM, its corresponding diffraction spots were absent from the LEED pattern. This is probably due to the presence of glide-planes, as previously described in the work of Bennett et al. [54].
3.2 Pd (~ 5 MLE) and Au (~ 1 MLE) double layer deposited on TiO2(110)-(1 × 1) at room temperature and the effects of annealing
As shown in Fig. 1 and previous work [24, 54, 55], Pd nanoparticles supported on TiO2(110) become encapsulated by an ultrathin TiOx layer when the Pd/TiO2 surface is annealed at > 873 K. Here we investigate whether incorporating Au into the Pd nanoparticles affects the encapsulation process. This might be expected because Au is more inert as a metal, and has a lower work function (~ 5.3 eV) and surface free energy (~ 1.51 Jm− 2) compared to Pd (5.6 eV and 2.01 Jm− 2). The experiment was performed by sequentially depositing 5 MLE of Pd and then 1 MLE of Au on TiO2(110) at room temperature, followed by annealing at 973 K for 20 min. AES spectra and STM images were recorded after each of the surface treatment steps. Figure 2 shows a number of AES spectra taken after each of the aforementioned surface treatment was performed, with the inset of which showing the relative intensities of the Auger transition signals of four different elements (O, Ti, Pd and Au), all normalized with respect to the intensity of the Ti(LMM) signal recorded from the clean TiO2(110) surface. As shown in Fig. 2, the spectrum taken from the clean TiO2(110) surface is characterized by three peaks, one of which is at 509 eV, the other two being at 384 and 416 eV. The peak at 509 eV corresponds to the KLL Auger transition of O, while the other two peaks at 384 and 416 eV correspond to the LMM and LMV Ti Auger transitions, respectively. Then 5 ML of Pd and 1 ML of Au were sequentially deposited onto the TiO2(110) substrate at room temperature (RT).
Auger electron spectra taken from four different surfaces: (Blue) clean TiO2(110), (Red) TiO2 after adsorption of 5 MLE Pd at 300 K; (Green), TiO2 after adsorption of 5 MLE Pd followed by 1 MLE Au at 300 K, and (black) the (5 MLE Pd + 1 MLE Au)/TiO2 surface after annealing at 973 K for 20 min. Inset: relative intensities of different Auger transition signals for the four different surfaces
Following the deposition of Pd (red), the intensities of the Auger transitions of Ti and O are reduced by a factor of around five, while three new peaks at 243, 279 and 330 eV appear. They are attributed to the MVV, MNV, and MNN Auger transitions of Pd. Deposition of Au (green) onto this surface leads to no detectable change in the peak intensities from Ti, O and Pd. However, a new peak appears at 73 eV kinetic energy, which we attribute to the NVV Auger transition of Au. The surface was then annealed at 973 K for 20 min. As shown in the resulting spectrum in Fig. 2 (black), this results in a noticeable recovery of the Auger signals from the TiO2(110) substrate, which we attribute to sintering of the Pd–Au layers. There is also a sharp decrease of the Au(NVV) signal (by 60%). In contrast, the Pd MNN only decreases by 40%. There are several thermally induced phenomena that can result in a greater decrease in the Au(NVV) signal: evaporation of Au from the surface, Au–Pd alloy formation, and diffusion of Au into the Pd multilayers. It has been reported that at < 1000 K Au does not evaporate from rutile TiO2 [31, 33] or alloy with Pd [2]. On this basis we attribute the decrease in the Auger signal of Au to the diffusion of Au into the Pd, which in turn leads to the formation of Au–Pd alloy core, Pd-shell supported nanoparticles. Although this interpretation is rather surprising when taking into account the fact that Au has a lower surface free energy (1.51 Jm− 2) compared to Pd (2.01 Jm− 2) [56], it has also been evidenced in a recent ion scattering spectroscopy (ISS) and X-ray photoelectron spectroscopy (XPS) study [42]. Moreover, in earlier work Au was found to diffuse into a Pd(111) single crystal [2]. In the ISS/XPS work, 5 ML Pd and 0.4 ML Au were sequentially deposited onto TiO2(110) at room temperature before annealing in the range 473–873 K. Although the Au ISS signal disappears at 773 K, XPS shows that the total amount of Au is maintained. This therefore suggests the formation of Au–Pd core Pd shell NPs. Further annealing to above 773 K leads to encapsulation of these bimetallic NPs with an atomically thick TiOx layer as a result of SMSI [42].
Here STM was also employed in order to obtain a clearer picture about the thermally induced material transport processes on this bimetallic system. As above, 5 ML Pd was first loaded onto the clean TiO2(110) substrate at RT. As shown in Fig. 3a, this resulted in a continuous Pd multilayer forming over the entire TiO2(110) substrate. A closer look at the Pd multilayer reveals that it is composed of a dense population of Pd grains, which were measured to have an averaged diameter of ~ 5 nm and height of ~ 1.2 nm. Subsequent deposition of 1 ML Au onto the Pd/TiO2(110) surface did not result in any noticeable change in the surface morphology as observed by STM (Fig. 3b). This is to be expected since the amount of Au added to the system only accounts for 16% of the total number of metal atoms deposited. Annealing the surface at 573 K for 20 min results in sintering (Fig. 3c). Increasing the anneal temperature to ~ 773 K leads to further dewetting of the Au–Pd multilayer, which exposes small areas of the TiO2 substrate (Fig. 3d). Annealing the surface to higher temperatures between 873 and 973 K facilities coalescence and/or Ostwald ripening, leading to the formation of separate, pseudo-hexagonal Au–Pd bimetallic nanoparticles supported on the TiO2(110) substrate (Fig. 3e, f). These nanoparticles have an averaged diameter of ~ 15 nm and height of ~ 2.5 nm.
50 × 50 nm2 STM images recorded from TiO2(110) after sequential deposition of a 5 MLE Pd and b 1MLE Au at 298 K, and c–f annealing at c 573, d 773, e 873 and f 973 K, for 20 min. at each temperature. g–i 6 × 3 nm2 atomically resolved STM images of the top-facet of one of the Au–Pd nanoparticles shown in d–f, respectively. All STM images were recorded at room temperature. Scan parameters: (a–f) VS, IT = + 1.2 V, 1.0 nA and g, h + 0.6 V, 3.0 nA
Corresponding high resolution STM images of the nanoparticles (Fig. 3h, i), reveal evidence of encapsulation with a zigzag-like TiOx layer. Indeed Fig. 3g, corresponding to an anneal temperature of ~ 773 K, reveals some traces of the development of the zigzag and pinwheel TiOx structures. This, along with the absence of any Au related features or discontinuities in the encapsulating oxide frameworks, supports the formation of Au/Pd-core, Pd-shell nanoparticles at temperatures of 773–973 K. The formation of this type of structure must arise from kinetic factors, as suggested previously [42, 57,58,59]. On the other hand, there also has to be an equilibrium concentration between the two metals within the Au–Pd mixture above which Pd no longer dissolves Au in its bulk. On this basis, we believe that Au/Pd core, Au-shell bimetallic NPs can be formed when the relative Pd/Au concentration is above such a threshold.
3.3 Pd (~ 3 MLE) and Au (~ 2 MLE) double layer deposited on TiO2(110)-(1 × 1) at room temperature and the effects of annealing
To test this idea, we sequentially deposited a reduced amount of Pd (3 MLE) and a doubled amount of Au (2 MLE) onto TiO2(110) at room temperature and investigated the effect of annealing as before. As shown in Fig. 4a, following the deposition of 3 MLE Pd at room temperature a large number of small Pd nanoparticles are formed across the TiO2(110) substrate. These Pd nanoparticles have a mean inter-particle distance of 0.8 ± 0.3 nm and average height of 0.9 ± 0.1 nm. Adsorption of 2 MLE of Au onto this surface leads to an increase in the average particle height to 1.2 ± 0.2 nm, while the mean inter-particle distance remains unchanged (Fig. 4b). The average height was measured from around 100 nanoparticles. This indicates that the Au atoms nucleate on the Pd nanoparticles, which is consistent with the behavior expected for a Pd(111) top facet of the nanoparticle [60].
a STM image of TiO2(110)-(1 × 1) after adsorption of 3 MLE Pd at 300 K. b As a, after additional adsorption of 2 MLE Au at 300 K. c–f STM images after the surface in b was sequentially annealed to c 573 K, d 773 K, e 873 K and f 973 K for 20 min. at each temperature. Inset in f high-resolution image recorded from the top facet of one of the Pd-core, Au-shell nanoparticles. Image sizes: a–f 50 × 50 nm2, inset of f 6 × 6 nm2. g LEED pattern taken from the surface in f at an electron energy of 87 eV. h Assignment of LEED pattern in g. Red squares mark the TiO2(110)-(1 × 1) unit cell, black hexagons mark the Au(111) unit cell. This gives a calculated nearest neighbour distance of 2.9 ± 0.1 Å. Scan parameters: a–f VS, IT = + 1.5 V, 0.1 nA and inset of f + 0.2 V, 5.0 nA
STM images (Fig. 4c–f) show that the morphology changes on annealing sequentially from 573 to 973 K are more gradual compared with the 5 MLE Pd: 1 MLE Au mixture described above. As the anneal temperature increases, small Au–Pd bimetallic nanoparticles ripen/coalesce, resulting in the formation of larger, more well-defined pseudo-hexagonal nanoparticles on the TiO2 support. The LEED pattern (Fig. 4g, h) from the surface annealed to 973 K is consistent with a (111) termination of the bimetallic nanoparticles. The corresponding nearest neighbor distance on the (111) top-facet is 2.9 ± 0.1 Å. This value is much larger than the lattice parameter of Pd(111) (2.75 Å), but very close to that of Au(111) (2.89 Å). This points to a Au(111) top layer of the nanoparticles.
The LEED pattern of the 973 K annealed surface shows no trace of the signature pattern of the pinwheel and zigzag structures, suggesting the absence of encapsulation. STM is consistent with this picture. As shown in the inset of Fig. 4f, the high resolution STM image taken from the top-facet of one of the bimetallic NPs exhibits an STM contrast that is much weaker in corrugation and very different from those of the pinwheel- and zigzag types of TiOx layer (Figs. 1d, 3g–i). We speculate that the image contrast arises from sub-surface alloy ordering. The absence of encapsulation, and Au(111) termination of the nanoparticles at this higher Au concentration is in line with our expectations. It is also consistent with earlier work where it was reported that a thick layer of Au covering Rh nanoparticles on TiO2(110) hinders the diffusion of Ti and O species onto the top facet up to ~ 900 K [32].
A summary of our conclusions regarding the effect of annealing Au/Pd nanoparticles is shown in Fig. 5. In the low Au-content regime (Fig. 5a. b), the nanoparticle has a bimetallic core and Pd shell, and as a result is encapsulated by the ordered TiOx structure after annealing. However, in the regime where the Au content is high (Fig. 5c, d), the nanoparticle core cannot dissolve more Au, and as a result the remaining Au atoms stay in the nanoparticle shell. The top facet appears to be essentially Au(111), which lowers the surface free energy and hinders SMSI of the nanoparticles.
Schematic ball models of the alloying and encapsulation processes of Au/Pd overlayers: a TiO2(110) afer adsorption of 5 MLE Pd followed by 1 MLE Au at 300 K. b As a, after sintering caused by annealing at 973 K for 20 min. c TiO2(110) after adsorption of 3 MLE Pd followed by 2 MLE Au at 300 K. d As c, after sintering caused by annealing at 973 K for 20 min
3.4 Template assisted adsorption, growth and thermal stability of Au nanoparticles on TiOx
One of the most intriguing aspects of ultrathin oxide layers is the possibility to use them as templates for metal adsorption to form uniform, periodically located metal nanoparticles on the oxide layer. This sort of behavior arises from the presence of more energetically favourable adsorption sites at the inter-corner regions (so-called pico-holes) of the pinwheel TiOx structure, which are not necessarily atomic vacancies in that oxide layer [45,46,47, 61]. Figure 6a shows an STM image of the pinwheel oxide layer following exposure of 0.1 MLE Au at RT. As indicated by the line plot in Fig. 6c, the Au species on the pinwheel structure have a measured diameter of 23 ± 5 Å and height of 1.1 ± 0.2 Å. In addition, by overlaying the image with a grid marking the lattice of the pinwheel structure (Fig. 6b), we can easily see that ~ 90% of the Au species adsorb at the pico-holes of the pinwheel structure, revealing a nearest neighbour distance of 1.6 ± 0.1 nm between the Au species. This suggests that the pico-holes of the pinwheel type oxide layer act as trapping sites for the impinging Au atoms, with bonding to the underlying metal at room temperature. Our findings hence agree extremely well with those reported by the Berkó group, who observed the Au species on the pinwheel type TiOx layer on the Rh(111) facet to exhibit very similar behavior at slightly higher deposition temperature (400–500 K) [31, 47]. Also, it is noteworthy that unlike the Au species on the pinwheel type TiOx layer, those nucleating on the zigzag type TiOx layer do not show any preference regarding the adsorption site.
a 10 × 5 nm2 STM image from the pinwheel encapsulating TiOx layer after adsorption of 0.1 MLE Au at 300 K (VS, IT = + 0.6 V, 1.0 nA). The encapsulation layer was formed on TiO2(110) after adsorption of 5 MLE Pd and 1 MLE Au followed by annealing to 973 K for 20 min. b As a, with white lines marking the unit cells of the pinwheel structure. These images reveal the pico-holes in the pinwheel structure to be the preferential adsorption sites for the Au clusters comprising 3–10 Au atoms. c Line plot taken along the line marked in a, showing a nearest neighbour distance of 1.6 ± 0.1 nm between the Au clusters
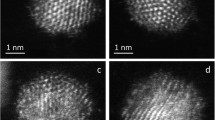
We annealed the Au nanoparticles on TiOx to explore their thermal stability. Figure 7a–e show high resolution STM images of the encapsulating TiOx layer formed on the Au–Pd bimetallic nanoparticles taken before (Fig. 7a) and after deposition of 0.1 MLE of Au at room temperature (Fig. 7b), and those taken after annealing at different temperatures (Fig. 7c–e). The insets in Fig. 7c–e show the morphology of the nanoparticles. As shown very clearly by the line-plots (i.e. line-plots X1 to X3 in Fig. 7f), the Au species retain more or less their original size and shape even after surface annealing at ~ 873 K, evidencing their apparent thermal stability. However, there is an apparent reduction in the number density of Au nanoparticles with increasing temperature, reaching 20% of initial density at 873 K before the nanoparticles completely disappear at 973 K. In contrast, Au nanoparticles directly bound to the TiO2 substrate are still present after annealing at 973 K (image not shown), which we attribute to their high desorption temperature from rutile [62].
The effect of annealing on the size and distribution of Au clusters deposited onto the TiOx encapsulation layer. a, b STM images recorded a before and b after deposition of 0.1 MLE Au on the encapsulating TiOx layer of the supported Au–Pd bimetallic nanoparticles shown in Fig. 6 at 300 K. c–e As d, after the surface was sequentially annealed at c 573, d 873 and e 973 K for 20 min. at each temperature. Image size: a, b 6 × 6 and c–e 8 × 8 nm2. Insets of a–e: the supported Au–Pd bimetallic nanoparticles on which the STM images in a–e were recorded. Average width and height of the NPs is 25 nm and 2.0 nm. Scan parameters: a–e VS, IT = + 0.1 V, 3.0 nA and insets of a–e + 1.3 V, 0.1 nA
In order to interpret our observation it is worth comparing our results with those by Berkó group [31]. In their work, 1 MLE Au was deposited at 500 K onto the TiOx layer formed on the (111) top facets of the Rh nanoparticles, with subsequent annealing up to 1050 K. In this earlier work it was observed that the Au species on the oxide layer are 3D in shape at lower temperatures. However, Ostwald-ripening starts to dominate as the temperature increases. At even higher temperature the Au nanoparticles start to spread out, forming a continuous, two-dimensional (2D) layer of Au across the entire oxide layer. This Au layer then disappears from the oxide layer at above 1000 K, which the authors attributed to the desorption of Au from the surface on the basis that the dissolution of Au into Rh is negligible [41]. In the present case where Au is a miscible metal with Pd, in addition to desorption of Au from the surface, the disappearance of the Au species from the oxide layer can also originate from the dissolution of the Au species into the bulk of the Au–Pd bimetallic nanoparticles [42].
4 Conclusions
Using STM, AES and LEED we have demonstrated that encapsulation of Au–Pd bimetallic nanoparticles on rutile TiO2 can be prevented by increasing the relative Au concentration. Such enrichment of Au leads to the formation of nanoparticles with a bimetallic core and Au-rich shell, which has a lower surface free energy and therefore is much less prone to SMSI.
We have also studied the room temperature adsorption behavior of Au on the pinwheel- and zigzag- type TiOx layers formed on the Au–Pd bimetallic nanoparticles. In STM, we found that the Au species preferentially adsorb at the pico-holes of the pinwheel structure, thus confirming the template-assisted growth mode as proposed by the Berkó group, while on the zigzag structure no preferential adsorption site of Au was detected. While the morphology of these Au species are stable at increasing temperatures, they vanish from the oxide layer completely at ~ 950 K, which we attribute to the dissolution of Au into the bulk, or their migration to the subsurface region of the nanoparticles.
References
Tominaga KI, Sasaki Y, Saito M et al (1994) Homogeneous Ru-Co bimetallic catalysis in CO2 hydrogenation: the formation of ethanol. J Mol Catal. doi:10.1016/0304-5102(93)E0287-Q
Baddeley CJ, Tikhov M, Hardacre C et al (1996) Ensemble effects in the coupling of acetylene to benzene on a bimetallic surface: A study WITH Pd{111}/Au. J Phys Chem 100:2189–2194. doi:10.1021/jp9517054
Chang C-R, Long B, Yang X-F, Li J (2015) Theoretical studies on the synergetic effects of Au–Pd bimetallic catalysts in the selective oxidation of methanol. J Phys Chem C 119:16072–16081. doi:10.1021/acs.jpcc.5b03965
Gao F, Goodman DW (2012) Pd–Au bimetallic catalysts: understanding alloy effects from planar models and (supported) nanoparticles. Chem Soc Rev 41:8009. doi:10.1039/c2cs35160a
Xu X, Szanyi J, Xu Q, Goodman DW (1994) Structural and catalytic properties of model silica- supported palladium catalysts: a comparison to single crystal surfaces. Catal Today 21:57–69. doi:10.1016/0920-5861(94)80034-0
Simonet J (2010) Gold doped by palladium building of Au–Pd electrodes showing exceptional capability for achieving electrocatalytic reductions. Electrochem commun 12:1475–1478. doi:10.1016/j.elecom.2010.08.010
Ponec V (2001) Alloy catalysts: the concepts. Appl Catal A Gen. doi:10.1016/S0926-860X(01)00828-6
Chen Q, Xin Y, Zhu X (2015) Au-Pd nanoparticles-decorated TiO2 nanobelts for photocatalytic degradation of antibiotic levofloxacin in aqueous solution. Electrochim Acta. doi:10.1016/j.electacta.2015.10.095
Su R, Tiruvalam R, Logsdail AJ et al (2014) Designer titania-supported au-pd nanoparticles for efficient photocatalytic hydrogen production. ACS Nano 8:3490–3497
Hahn C, Abram DN, Hansen HA et al (2015) Synthesis of thin film AuPd alloys and their investigation for electrocatalytic CO2 reduction. J Mater Chem A 3:20185–20194. doi:10.1039/C5TA04863J
Greeley J, Jaramillo TF, Bonde J et al (2006) Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat Mater. doi:10.1038/nmat1752
Chen MS, Luo K, Wei T et al (2006) The nature of the active site for vinyl acetate synthesis over Pd-Au. Catal Today 117:37–45. doi:10.1016/j.cattod.2006.05.001
Gleich B, Ruff M, Behm RJ (1997) Correlation between local substrate structure and local chemical properties: CO adsorption on well-defined bimetallic surfaces. Surf Sci 386:48–55. doi:10.1016/S0039-6028(97)00302-6
Lucci FR, Darby MT, Mattera MFG et al (2016) Controlling hydrogen activation, spillover, and desorption with Pd-Au Single-Atom Alloys. J Phys Chem Lett. doi:10.1021/acs.jpclett.5b02400
Zhu B, Thrimurthulu G, Delannoy L et al (2013) Evidence of Pd segregation and stabilization at edges of AuPd nano-clusters in the presence of CO: a combined DFT and DRIFTS study. J Catal 308:272–281. doi:10.1016/j.jcat.2013.08.022
Yu W-Y, Zhang L, Mullen GM et al (2015) Effect of annealing in oxygen on alloy structures of Pd–Au bimetallic model catalysts. Phys Chem Chem Phys 17:20588–20596. doi:10.1039/C5CP03515E
Fu Q, Wagner T (2007) Interaction of nanostructured metal overlayers with oxide surfaces. Surf Sci Rep 62:431–498. doi:10.1016/j.surfrep.2007.07.001
Zaleska-Medynska A, Marchelek M, Diak M, Grabowska E (2016) Noble metal-based bimetallic nanoparticles: the effect of the structure on the optical, catalytic and photocatalytic properties. Adv Colloid Interface Sci 229:80–107. doi:10.1016/j.cis.2015.12.008
Alshammari A, Kalevaru V, Martin A (2016) Bimetallic catalysts containing gold and palladium for environmentally important reactions. Catalysts. doi:10.3390/catal6070097
Chen M (2005) The promotional effect of gold in catalysis by palladium-gold. Science 310:291–293. doi:10.1126/science.1115800
Enache DI (2006) Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalysts. Science 311:362–365. doi:10.1126/science.1120560
Edwards JK, Solsona BE, Landon P et al (2005) Direct synthesis of hydrogen peroxide from H2 and O2 using TiO2-supported Au-Pd catalysts. J Catal 236:69–79. doi:10.1016/j.jcat.2005.09.015
Yang G, Chen D, Lv P et al (2016) Core-shell Au-Pd nanoparticles as cathode catalysts for microbial fuel cell applications. Sci Rep. doi:10.1038/srep35252
Bowker M, Sharpe R (2015) Pd deposition on TiO2 (110) and nanoparticle encapsulation. Catal Struct React 1:140–145. doi:10.1179/2055075815Y.0000000008
Majzik Z, Balázs N, Berkó A (2011) Ordered SMSI decoration layer on Rh nanoparticles grown on TiO2 (110) surface. J Phys Chem C 115:9535–9544. doi:10.1021/jp111319n
Atanasov I, Hou M (2009) Equilibrium ordering properties of Au-Pd alloys and nanoalloys. Surf Sci 603:2639–2651. doi:10.1016/j.susc.2009.06.018
Christensen A, Ruban A, Stoltze P et al (1997) Phase diagrams for surface alloys. Phys Rev B 56:5822–5834. doi:10.1103/PhysRevB.56.5822
Mejía-Rosales SJ, Fernández-Navarro C, Pérez-Tijerina E et al (2007) On the structure of Au/Pd bimetallic nanoparticles. J Phys Chem C 111:1256–1260. doi:10.1021/jp066328h
Okamoto H, Massalski TB (1985) The Au–Pd (Gold-Palladium) system. Bull Alloy Phase Diagr 6:229–235. doi:10.1007/BF02880404
Yudanov IV, Neyman KM (2010) Stabilization of Au at edges of bimetallic PdAu nanocrystallites. Phys Chem Chem Phys 12:5094. doi:10.1039/b927048e
Gubó R, Óvári L, Kónya Z, Berkó A (2014) Growth of gold on a pinwheel TiO ∼1.2 encapsulation film prepared on rhodium nanocrystallites. Langmuir 30:14545–14554. doi:10.1021/la503756c
Óvári L, Berkó A, Gubó R et al (2014) Effect of a gold cover layer on the encapsulation of rhodium by titanium oxides on titanium dioxide(110). J Phys Chem C 118:12340–12352. doi:10.1021/jp502748a
Park JB, Conner SF, Chen DA (2008) Bimetallic Pt-Au clusters on TiO2(110): growth, surface composition, and metal-support interactions. J Phys Chem C. doi:10.1021/jp076027n
Yu W, Porosoff MD, Chen JG (2012) Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem Rev 112:5780–5817. doi:10.1021/cr300096b
Bowker M (2007) Catalysis resolved using scanning tunnelling microscopy. Chem Soc Rev 36:1656. doi:10.1039/b412139m
Shi XY, Zhang W, Zhang C et al (2016) Real-space observation of strong metal-support interaction: state-of-the-art and what’s the next. J Microsc 262:203–215. doi:10.1111/jmi.12366
Berko A, Ulrych I, Prince KC (1998) Encapsulation of Rh nanoparticles supported on TiO2(110)-(1 × 1) surface: XPS and STM studies. J Phys Chem B 102:3379–3386. doi:10.1021/jp973255g
Bowker M, Stone P, Bennett R, Perkins N (2002) CO adsorption on a Pd/TiO2(1 1 0) model catalyst. Surf Sci 497:155–165. doi:10.1016/S0039-6028(01)01640-5
Centi G, Fornasiero P, Graziani M et al (2001) Enhancement of the low temperature activity in NO reduction in lean conditions by SMSI effect in Pt/CeO2-ZrO2 on alumina catalyst. Top Catal 16:173–180. doi:10.1023/A:1016611704769
Wu C, Marshall MSJ, Castell MR (2011) Surface structures of ultrathin TiOx films on Au(111). J Phys Chem C 115:8643–8652. doi:10.1021/jp111385n
Óvári L, Berkó A, Vári G et al (2016) The growth and thermal properties of Au deposited on Rh(111): formation of an ordered surface alloy. Phys Chem Chem Phys 18:25230–25240. doi:10.1039/C6CP02128J
Sharpe R, Counsell J, Bowker M (2017) Pd segregation to the surface of Au on Pd(111) and on Pd/TiO2(110). Surf Sci. doi:10.1016/j.susc.2016.10.005
Xu M, Liang T, Shi M, Chen H (2013) Graphene-like two-dimensional materials. Chem Rev. doi:10.1021/cr300263a
Gavioli L, Cavaliere E, Agnoli S et al (2011) Template-assisted assembly of transition metal nanoparticles on oxide ultrathin films. Prog Surf Sci 86:59–81. doi:10.1016/j.progsurf.2011.02.001
Artiglia L, Cavaliere E, Gavioli L, Rizzi GA (2015) Interaction of iron with a wagon wheel-like ultrathin TiOx film grown on Pt(111). Phys Chem Chem Phys 17:18055–18062. doi:10.1039/C5CP01931A
Barcaro G, Cavaliere E, Artiglia L et al (2012) Building principles and structural motifs in TiOx ultrathin films on a (111) substrate. J Phys Chem C 116:13302–13306. doi:10.1021/jp303730j
Mutombo P, Gubó R, Berkó A (2016) Interaction of gold with a pinwheel TiO∼1.2 film formed on Rh(111) facet: STM and DFT studies. J Phys Chem C 120:12917–12923. doi:10.1021/acs.jpcc.6b03959
Horcas I, Fernández R, Gómez-Rodríguez JM et al (2007) WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev Sci Instrum. doi:10.1063/1.2432410
Diebold U (2003) The surface science of titanium dioxide. Surf Sci Rep 48:53–229. doi:10.1016/S0167-5729(02)00100-0
Bikondoa O, Pang CL, Ithnin R et al (2006) Direct visualization of defect-mediated dissociation of water on TiO2(110). Nat Mater 5:189–192. doi:10.1038/nmat1592
Pang CL, Lindsay R, Thornton G (2013) Structure of clean and adsorbate-covered single-crystal rutile TiO2 surfaces. Chem Rev 113:3887–3948. doi:10.1021/cr300409r
Solymosi F (1985) Comments on electronic effects in strong metal-support interactions on titania-deposited metal catalysts. J Catal 94:581–585. doi:10.1016/0021-9517(85)90226-X
Tauster SJ, Fung SC (1978) Strong metal-support interactions: occurrence among the binary oxides of groups IIA-VB. J Catal. doi:10.1016/0021-9517(78)90182-3
Bennett RA, Pang CL, Perkins N et al (2002) Surface structures in the SMSI State; Pd on (1 × 2) reconstructed TiO2 (110). J Phys Chem B 106:4688–4696. doi:10.1021/jp0138328
Bowker M, Fourré E (2008) Direct interactions between metal nanoparticles and support: STM studies of Pd on TiO2(110). Appl Surf Sci doi. 10.1016/j.apsusc.2008.01.014
Vitos L, Ruban AV, Skriver HL, Kollár J (1998) The surface energy of metals. Surf Sci 411:186–202. doi:10.1016/S0039-6028(98)00363-X
Lee AF, Baddeley CJ, Hardacre C et al (1995) Structural and catalytic properties of novel Au/Pd bimetallic colloid particles: EXAFS, XRD, and acetylene coupling. J Phys Chem 99
Lee YW, Kim M, Kim ZH, Han SW (2009) One-step synthesis of Au@Pd core-shell nanooctahedron. J Am Chem Soc 131:17036–17037. doi:10.1021/ja905603p
Chantry RL, Atanasov I, Siriwatcharapiboon W et al (2013) An atomistic view of the interfacial structures of AuRh and AuPd nanorods. Nanoscale 5:7452. doi:10.1039/c3nr02560h
Pimpinelli A, Villain J (1998) Physics of crystal growth. Cambridge University Press, Cambridge. doi:10.1017/CBO9780511622526
Barcaro G, Fortunelli A (2009) Adsorption and diffusion of fe on a titania ultrathin film. J Phys Chem A 113:14860–14866. doi:10.1021/jp904998c
Óvári L, Berko A, Balázs N et al (2010) Formation of Rh-Au core-shell nanoparticles on TiO2(110) surface studied by STM and LEIS. Langmuir 26:2167–2175. doi:10.1021/la902674u
Acknowledgements
We acknowledge fruitful discussions with Professor Michael Bowker. The UCL group is supported by a European Research Council Advanced grant ENERGYSURF, and the Royal Society through a Wolfson Research Merit Award. The Szeged group is supported by research grant NKFIH-OTKA-120115. This collaboration was funded by a European Cooperation in Science and Technology Action: ECOST-STSM-CM1104-030815-063692. The ELI-ALPS project (GINOP-2.3.6-15-2015-00001) is supported by the European Union and co-financed by the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gubó, R., Yim, C.M., Allan, M. et al. Variation of SMSI with the Au:Pd Ratio of Bimetallic Nanoparticles on TiO2(110). Top Catal 61, 308–317 (2018). https://doi.org/10.1007/s11244-017-0854-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-017-0854-5