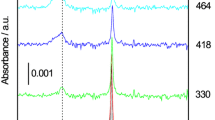
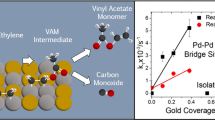
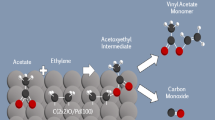
Abstract
The reaction pathways for the synthesis of vinyl acetate monomer (VAM) are explored on model palladium and gold–palladium alloy single crystal catalysts by combining experiments carried out in ultrahigh vacuum together with density functional theory calculations and Monte Carlo simulations. Previous work by Goodman has shown that both pure palladium and gold–palladium alloys catalyze VAM formation at high pressures, thereby paving the way for fundamental studies of the pathways for this reaction. The coverages of the reactants and products on the surface were found to play an important role in controlling both the reaction pathways and the selectivity. The high coverages on the catalyst under reaction conditions favor bond-forming reactions while inhibiting bond-breaking reactions. On Pd(111), the reaction is initiated by the coupling of ethylene and surface acetate species to form an acetoxyethyl-palladium intermediate, a bond-forming reaction. The high coverages also act to control the selectivity since VAM is stabilized on the crowded surface. The gold in model Au/Pd(111) and Au/Pd(100) alloys gold preferentially segregates to the surface. In the case of Au/Pd(111) alloys, there is a slightly repulsive interaction between the gold and palladium atoms, resulting in a larger proportion of isolated palladium sites than would be expected if they were randomly distributed, while the longer-range interactions on Au/Pd(100) lead to the formation of ordered surface structures and the existence of isolated palladium sites for gold coverages greater than 0.5 ML. Higher coverages of Au on the Au/Pd(111) and Au/Pd(100) alloys decrease the population of bridging Pd sites and thus increase Pd site isolation. This eliminates the larger Pd ensembles that that lead to the decomposition of VAM and ethylene thus increasing the reaction selectivity and weakens the adsorption of ethylene and acetate which enhances the rate of reaction. Higher coverages of Au, however, also suppress the activation of O2 which decrease the rate of acid deprotonation thus resulting in optimal Au/Pd compositions.






















Similar content being viewed by others
References
Gao F, Wang Y, Goodman DW (2009) CO oxidation over AuPd(100) from ultrahigh vacuum to near-atmospheric pressures: CO adsorption-induced surface segregation and reaction kinetics. J Phys Chem C 113(33):14993–15000. doi:10.1021/jp9053132
Gao F, Wang Y, Goodman DW (2009) CO oxidation over AuPd(100) from ultrahigh vacuum to near-atmospheric pressures: the critical role of contiguous Pd atoms. J Am Chem Soc 131(16):5734–5735. doi:10.1021/ja9008437
Beck A, Horváth A, Schay Z, Stefler G, Koppány Z, Sajó I, Geszti O, Guczi L (2007) Sol derived gold–palladium bimetallic nanoparticles on TiO2: structure and catalytic activity in CO oxidation. Top Catal 44(1–2):115–121. doi:10.1007/s11244-007-0284-x
Edwards JK, Hutchings GJ (2008) Palladium and gold–palladium catalysts for the direct synthesis of hydrogen peroxide. Angew Chem Int Ed 47(48):9192–9198. doi:10.1002/anie.200802818
Edwards JK, Ntainjua NE, Carley AF, Herzing AA, Kiely CJ, Hutchings GJ (2009) Direct Synthesis of H2O2 from H2 and O2 over gold, palladium, and gold–palladium catalysts supported on acid-pretreated TiO2. Angew Chem Int Ed 48(45):8512–8515. doi:10.1002/anie.200904115
Edwards JK, Solsona B, N EN, Carley AF, Herzing AA, Kiely CJ, Hutchings GJ (2009) Switching off hydrogen peroxide hydrogenation in the direct synthesis process. Science 323(5917):1037–1041. doi:10.1126/science.1168980
Edwards JK, Solsona BE, Landon P, Carley AF, Herzing A, Kiely CJ, Hutchings GJ (2005) Direct synthesis of hydrogen peroxide from H2 and O2 using TiO2-supported Au–Pd catalysts. J Catal 236(1):69–79. doi:10.1016/j.jcat.2005.09.015
Edwards JK, Thomas A, Solsona BE, Landon P, Carley AF, Hutchings GJ (2007) Comparison of supports for the direct synthesis of hydrogen peroxide from H2 and O2 using Au–Pd catalysts. Catal Today 122(3–4):397–402. doi:10.1016/j.cattod.2007.01.046
Solsona BE, Edwards JK, Landon P, Carley AF, Herzing A, Kiely CJ, Hutchings GJ (2006) Direct synthesis of hydrogen peroxide from H2 and O2 using Al2O3 supported Au–Pd catalysts. Chem Mater 18(11):2689–2695. doi:10.1021/cm052633o
Enache DI, Barker D, Edwards JK, Taylor SH, Knight DW, Carley AF, Hutchings GJ (2007) Solvent-free oxidation of benzyl alcohol using titania-supported gold–palladium catalysts: effect of Au–Pd ratio on catalytic performance. Catal Today 122(3–4):407–411. doi:10.1016/j.cattod.2007.01.003
Edwards J, Landon P, Carley AF, Herzing AA, Watanabe M, Kiely CJ, Hutchings GJ (2007) Nanocrystalline gold and gold–palladium as effective catalysts for selective oxidation. J Mater Res 22(04):831–837. doi:10.1557/jmr.2007.0117
Chen MS, Kumar D, Yi CW, Goodman DW (2005) The promotional effect of gold in catalysis by palladium–gold. Science 310(5746):291–293. doi:10.1126/science.1115800
Chen MS, Luo K, Wei T, Yan Z, Kumar D, Yi CW, Goodman DW (2006) The nature of the active site for vinyl acetate synthesis over Pd–Au. Catal Today 117(1–3):37–45. doi:10.1016/j.cattod.2006.05.001
Han YF, Kumar D, Goodman DW (2005) Particle size effects in vinyl acetate synthesis over Pd/SiO2. J Catal 230(2):353–358. doi:10.1016/j.jcat.2004.12.018
Han YF, Wang JH, Kumar D, Yan Z, Goodman DW (2005) A kinetic study of vinyl acetate synthesis over Pd-based catalysts: kinetics of vinyl acetate synthesis over Pd–Au/SiO2 and Pd/SiO2 catalysts. J Catal 232(2):467–475. doi:10.1016/j.jcat.2005.04.001
Kumar D, Chen MS, Goodman DW (2007) Synthesis of vinyl acetate on Pd-based catalysts. Catal Today 123(1–4):77–85. doi:10.1016/j.cattod.2007.01.050
Provine WD, Mills PL, Lerou JJ (1996) Discovering the role of Au and KOAc in the catalysis of vinyl acetate synthesis. Stud Surf Sci Catal 101:191–200. doi:10.1016/s0167-2991(96)80229-1
Colling PM, Johnson LR, Nicolau I (1996) Palladium–gold catalyst for vinyl acetate production. US Patent
Horning L, Wunder F, Quadflieg T (1967) Process for preparing vinyl acetates. US Patent
Robinson RE (1965) Process for preparing esters. US Patent
Sinfelt JH (1983) Bimetallic catalysts: discoveries, concepts, and applications. Wiley, New York
Dowden DA, Reynolds PW (1950) Some reactions over alloy catalysts. Discuss Faraday Soc 8:184–190
Schwab G-M (1950) Alloy catalysts in dehydrogenation. Discuss Faraday Soc 8:166–171
Han P, Axnanda S, Lyubinetsky I, Goodman DW (2007) Atomic-scale assembly of a heterogeneous catalytic site. J Am Chem Soc 129(46):14355–14361. doi:10.1021/ja074891n
Wei T, Kumar D, Chen MS, Luo K, Axnanda S, Lundwall M, Goodman DW (2008) Vinyl acetate synthesis over model Pd–Sn bimetallic catalysts. J Phys Chem C 112(22):8332–8337. doi:10.1021/jp8005266
Woodruff DP (2002) Surface alloys and alloy surfaces. Elsevier, Amsterdam
Rodriguez J (1996) Physical and chemical properties of bimetallic surfaces. Surf Sci Rep 24(7–8):223–287. doi:10.1016/0167-5729(96)00004-0
Gao F, Goodman DW (2012) Pd–Au bimetallic catalysts: understanding alloy effects from planar models and (supported) nanoparticles. Chem Soc Rev 41(24):8009–8020
Calaza F, Stacchiola D, Neurock M, Tysoe WT (2010) Coverage effects on the palladium-catalyzed synthesis of vinyl acetate: comparison between theory and experiment. J Am Chem Soc 132(7):2202–2207. doi:10.1021/ja907061m
Neurock M, Venkataraman PS, van Santen RA (2000) The importance of transient states at higher coverages in catalytic reactions. J Am Chem Soc 122(6):1150–1153
Neurock M (2003) Perspectives on the first principles elucidation and the design of active sites. J Catal 216(1–2):73–88. doi:10.1016/s0021-9517(02)00115-x
Neurock M (2008) Theory aided catalyst design in design of heterogeneous catalysts: new approaches based on synthesis, characterization and modelling. VCH-Wiley, Weinheim
Hansen N, Heyden A, Bell AT, Keil FJ (2007) A reaction mechanism for the nitrous oxide decomposition on binuclear oxygen bridged iron sites in Fe-ZSM-5. J Phys Chem C 111(5):2092–2101. doi:10.1021/jp065574q
Heyden A, Hansen N, Bell AT, Keil FJ (2006) Nitrous oxide decomposition over Fe-ZSM-5 in the presence of nitric oxide: a comprehensive DFT study. J Phys Chem B 110(34):17096–17114. doi:10.1021/jp062814t
Samanos B, Boutry P, Montarnal R (1971) The mechanism of vinyl acetate formation by gas-phase catalytic ethylene acetoxidation. J Catal 23(1):19–30. doi:10.1016/0021-9517(71)90019-4
Moiseev II, Vargaftik MN (1960) Izr Akad Nauk SSSR (Engl Trans 133)
Moiseev II, Vargaftik MN (1992) Perspectives in catalysis, chemistry for the 21st Century. Blackwell Scientific, Oxford
Bronsted JN (1928) Acid and basic catalysis. Chem Rev 5(3):231–338. doi:10.1021/cr60019a001
Evans MG, Polanyi M (1938) Inertia and driving force of chemical reactions. Trans Faraday Soc 34:11–24
Logadottir A, Rod TH, Nørskov JK, Hammer B, Dahl S, Jacobsen CJH (2001) The Brønsted–Evans–Polanyi relation and the volcano plot for ammonia synthesis over transition metal catalysts. J Catal 197(2):229–231. doi:10.1006/jcat.2000.3087
Pallassana V, Neurock M (2000) Electronic factors governing ethylene hydrogenation and dehydrogenation activity of pseudomorphic PdML/Re(0001), PdML/Ru(0001), Pd(111), and PdML/Au(111) surfaces. J Catal 191(2):301–317. doi:10.1006/jcat.1999.2724
Santen RA, Neurock M, Shetty SG (2009) Reactivity theory of transition-metal surfaces: a Brønsted–Evans–Polanyi linear activation energy–free-energy analysis. Chem Rev 110(4):2005–2048. doi:10.1021/cr9001808
Santen RA, Neurock M (2006) Molecular heterogeneous catalysis: a conceptual and computational approach. Wiley-VCH, Weinheim
Aschoff M, Speller S, Kuntze J, Heiland W, Platzgummer E, Schmid M, Varga P, Baretzky B (1998) Unreconstructed Au(100) monolayers on a Au3Pd(100) single-crystal surface. Surf Sci 415(3):L1051–L1054. doi:10.1016/S0039-6028(98)00564-0
Piccolo L, Piednoir A, Bertolini J-C (2005) Pd–Au single-crystal surfaces: segregation properties and catalytic activity in the selective hydrogenation of 1,3-butadiene. Surf Sci 592(1–3):169–181. doi:10.1016/j.susc.2005.07.005
Yi CW, Luo K, Wei T, Goodman DW (2005) The composition and structure of Pd–Au surfaces. J Phys Chem B 109(39):18535–18540. doi:10.1021/jp053515r
Wei T, Wang J, Goodman DW (2007) Characterization and chemical properties of Pd–Au alloy surfaces. J Phys Chem C 111(25):8781–8788. doi:10.1021/jp067177l
Weissman-Wenocur DL, Stefan PM, Pate BB, Shek ML, Lindau I, Spicer WE (1983) Photoemission study of Au overlayers on Pd(111) and the formation of a Pd–Au(111) alloy surface. Phys Rev B 27(6):3308–3317
Baddeley CJ, Tikhov M, Hardacre C, Lomas JR, Lambert RM (1996) Ensemble effects in the coupling of acetylene to benzene on a bimetallic surface: a study with Pd{111}/Au. J Phys Chem 100(6):2189–2194. doi:10.1021/jp9517054
Lee AF, Baddeley CJ, Hardacre C, Ormerod RM, Lambert RM, Schmid G, West H (1995) Structural and catalytic properties of novel Au/Pd bimetallic colloid particles: EXAFS, XRD, and acetylene coupling. J Phys Chem 99(16):6096–6102. doi:10.1021/j100016a053
Baddeley CJ, Barnes CJ, Wander A, Ormerod RM, King DA, Lambert RM (1994) Surface crystallography of three catalytically important structures in the Au{111}–Pd system. Surf Sci 314(1):1–12. doi:10.1016/0039-6028(94)90208-9
Okamoto H, Massalski TB (1985) The Au–Pd (gold–palladium) system. Bull Alloy Phase Diagr 6(3):229–235. doi:10.1007/bf02880404
Li Z, Furlong O, Calaza F, Burkholder L, Poon HC, Saldin D, Tysoe WT (2008) Surface segregation of gold for Au/Pd(111) alloys measured by low-energy electron diffraction and low-energy ion scattering. Surf Sci 602(5):1084–1091. doi:10.1016/j.susc.2008.01.019
Boscoboinik JA, Plaisance C, Neurock M, Tysoe WT (2008) Monte Carlo and density functional theory analysis of the distribution of gold and palladium atoms on Au/Pd(111) alloys. Phys Rev B 77(4):1–6. doi:10.1103/PhysRevB.77.045422
Garvey M, Boscoboinik JA, Burkholder L, Walker J, Plaisance C, Neurock M, Tysoe WT (2011) The structure of the Au/Pd(100) alloy surface. J Phys Chem C 116(7):4692–4697
James J, Saldin DK, Zheng T, Tysoe WT, Sholl DS (2005) Structure and binding site of acetate on Pd(111) determined using density functional theory and low energy electron diffraction. Catal Today 105(1):74–77. doi:10.1016/j.cattod.2005.04.009
Haley RD, Tikhov MS, Lambert RM (2001) The surface chemistry of acetic acid on Pd{111}. Catal Lett 76(3–4):125–130. doi:10.1023/a:1012330230543
Hansen E, Neurock M (2001) First-principles based kinetic simulations of acetic acid temperature programmed reaction on Pd(111). J Phys Chem B 105(38):9218–9229. doi:10.1021/jp0103427
Davis JL, Barteau MA (1991) Reactions of carboxylic-acids on the Pd(111)-(2 × 2)O surface: multiple roles of surface oxygen atoms. Surf Sci 256(1–2):50–66. doi:10.1016/0039-6028(91)91199-8
Davis JL, Barteau MA (1989) Hydrogen bonding in carboxylic acid adlayers on Pd(111): evidence for catemer formation. Langmuir 5(6):1299–1309. doi:10.1021/la00090a004
Gates JA, Kesmodel LL (1983) Thermal evolution of acetylene and ethylene on Pd(111). Surf Sci 124(1):68–86. doi:10.1016/0039-6028(83)90336-9
Gates JA, Kesmodel LL (1982) EELS analysis of the low temperature phase of ethylene chemisorbed on Pd(111). Surf Sci 120(2):L461–L467. doi:10.1016/0039-6028(82)90141-8
Kaltchev M, Thompson AW, Tysoe WT (1997) Reflection–absorption infrared spectroscopy of ethylene on palladium(111) at high pressure. Surf Sci 391(1–3):145–149. doi:10.1016/s0039-6028(97)00475-5
Stacchiola D, Burkholder L, Tysoe WT (2002) Ethylene adsorption on Pd(111) studied using infrared reflection–absorption spectroscopy. Surf Sci 511(1–3):215–228. doi:10.1016/s0039-6028(02)01498-x
Wang LP, Tysoe WT, Ormerod RM, Lambert RM, Hoffmann H, Zaera F (1990) Determination of the bonding and orientation of ethylene on palladium(111) by near-edge X-ray absorption fine structure and photoelectron spectroscopy. J Phys Chem 94(10):4236–4239. doi:10.1021/j100373a066
Stacchiola D, Calaza F, Burkholder L, Schwabacher AW, Neurock M, Tysoe WT (2005) Elucidation of the reaction mechanism for the palladium-catalyzed synthesis of vinyl acetate. Angew Chem-Int Ed 44(29):4572–4574. doi:10.1002/anie.200500782
Stacchiola D, Calaza F, Burkholder L, Tysoe WT (2004) Vinyl acetate formation by the reaction of ethylene with acetate species on oxygen-covered Pd(111). J Am Chem Soc 126(47):15384–15385. doi:10.1021/ja044641w
Rubinstein RY (1981) Simulation and the Monte Carlo method. Wiley, New York
Stacchiola D, Tysoe WT (2003) The effect of subsurface hydrogen on the adsorption of ethylene on Pd(111). Surf Sci 540(2–3):L600–L604. doi:10.1016/s0039-6028(03)00848-3
Tysoe WT, Nyberg GL, Lambert RM (1984) Structural, kinetic, and reactive properties of the palladium(111)–ethylene system. J Phys Chem 88(10):1960–1963. doi:10.1021/j150654a009
Wytenberg WJ, Lambert RM (1992) A long-lived aluminium evaporation source for controlled, reproducible deposition of clean ultra-thin films under UHV conditions. J Vac Sci Technol A 10(6):3597–3598
Kresse G, Hafner J (1993) Ab initio molecular dynamics for liquid metals. Phys Rev B 47(1):558–561
Kresse G, Furthmüller J (1996) Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6(1):15–50. doi:10.1016/0927-0256(96)00008-0
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54(16):11169–11186
Laasonen K, Car R, Lee C, Vanderbilt D (1991) Implementation of ultrasoft pseudopotentials in ab initio molecular dynamics. Phys Rev B 43(8):6796–6799
Laasonen K, Pasquarello A, Car R, Lee C, Vanderbilt D (1993) Car–Parrinello molecular dynamics with Vanderbilt ultrasoft pseudopotentials. Phys Rev B 47(16):10142–10153
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Perdew JP, Burke K, Wang Y (1996) Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys Rev B 54(23):16533–16539
Methfessel M, Paxton AT (1989) High-precision sampling for Brillouin-zone integration in metals. Phys Rev B 40(6):3616–3621
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50(24):17953–17979
Kawasaki K (1966) Diffusion constants near the critical point for time-dependent ising models. I. Phys Rev 145(1):224–230
Pendry JB (1974) Low energy electron diffraction: the theory and its application to determination of surface structure. Academic Press, London
Maroun F, Ozanam F, Magnussen OM, Behm RJ (2001) The role of atomic ensembles in the reactivity of bimetallic electrocatalysts. Science 293(5536):1811–1814. doi:10.1126/science.1061696
Li Z, Gao F, Furlong O, Tysoe WT (2010) Adsorption of carbon monoxide Au/Pd(100) alloys in ultrahigh vacuum: identification of adsorption sites. Surf Sci 604(2):136–143. doi:10.1016/j.susc.2009.10.031
Conrad H, Ertl G, Küppers J, Latta EE (1977) Interaction of NO and O2 with Pd(111) surfaces. II. Surf Sci 65(1):245–260. doi:10.1016/0039-6028(77)90305-3
Klötzer B, Hayek K, Konvicka C, Lundgren E, Varga P (2001) Oxygen-induced surface phase transformation of Pd(111): sticking, adsorption and desorption kinetics. Surf Sci 482–485(Part 1):237–242. doi:10.1016/S0039-6028(01)00750-6
Eichler A, Mittendorfer F, Hafner J (2000) Precursor-mediated adsorption of oxygen on the (111) surfaces of platinum-group metals. Phys Rev B 62(7):4744–4755
Blanco JM, González C, Jelínek P, Ortega J, Flores F, Pérez R, Rose M, Salmeron M, Méndez J, Wintterlin J, Ertl G (2005) Origin of contrast in STM images of oxygen on Pd(111) and its dependence on tip structure and tunneling parameters. Phys Rev B 71(11):113402
Boscoboinik JA, Calaza FC, Garvey MT, Tysoe WT (2010) Identification of adsorption ensembles on bimetallic alloys. J Phys Chem C 114(4):1875–1880. doi:10.1021/jp9078794
Xu Y, Mavrikakis M (2003) Adsorption and dissociation of O2 on gold surfaces: effect of steps and strain. J Phys Chem B 107(35):9298–9307. doi:10.1021/jp034380x
Deng X, Min BK, Guloy A, Friend CM (2005) Enhancement of O2 dissociation on Au(111) by adsorbed oxygen: implications for oxidation catalysis. J Am Chem Soc 127(25):9267–9270. doi:10.1021/ja050144j
Li Z, Gao F, Tysoe WT (2010) Carbon monoxide oxidation over Au/Pd(100) model alloy catalysts. J Phys Chem C 114(40):16909–16916. doi:10.1021/jp911374u
Joshi AM, Delgass WN, Thomson KT (2007) Investigation of gold–silver, gold–copper, and gold–palladium dimers and trimers for hydrogen peroxide formation from H2 and O2. J Phys Chem C 111(20):7384–7395. doi:10.1021/jp066828a
Todorovic R, Meyer RJ (2011) A comparative density functional theory study of the direct synthesis of H2O2 on Pd, Pt and Au surfaces. Catal Today 160(1):242–248. doi:10.1016/j.cattod.2010.07.011
Beebe TP, Yates JT (1986) An in situ infrared spectroscopic investigation of the role of ethylidyne in the ethylene hydrogenation reaction on palladium/alumina. J Am Chem Soc 108(4):663–671. doi:10.1021/ja00264a016
Kesmodel LL, Dubois LH, Somorjai GA (1978) Dynamical LEED study of C2H2 and C2H4 chemisorption on Pt(111): evidence for the ethylidyne group. Chem Phys Lett 56(2):267–271. doi:10.1016/0009-2614(78)80236-x
Cremer PS, Su X, Shen YR, Somorjai GA (1996) Ethylene hydrogenation on Pt(111) monitored in situ at high pressures using sum frequency generation. J Am Chem Soc 118(12):2942–2949. doi:10.1021/ja952800t
Davis SM, Zaera F, Gordon BE, Somorjai GA (1985) Radiotracer and thermal desorption studies of dehydrogenation and atmospheric hydrogenation of organic fragments obtained from [14C]ethylene chemisorbed over Pt(111) surfaces. J Catal 92(2):240–246. doi:10.1016/0021-9517(85)90258-1
Stuve EM, Madix RJ (1985) Bonding and dehydrogenation of ethylene on palladium metal. Vibrational spectra and temperature-programed reaction studies on palladium(100). J Phys Chem 89(1):105–112. doi:10.1021/j100247a026
Zaera F, Somorjai GA (1984) Hydrogenation of ethylene over platinum (111) single-crystal surfaces. J Am Chem Soc 106(8):2288–2293. doi:10.1021/ja00320a013
Calaza F, Gao F, Li Z, Tysoe WT (2007) The adsorption of ethylene on Au/Pd(111) alloy surfaces. Surf Sci 601(3):714–722. doi:10.1016/j.susc.2006.10.039
Hansen EW, Neurock M (2000) First-principles-based Monte Carlo simulation of ethylene hydrogenation kinetics on Pd. J Catal 196(2):241–252. doi:10.1006/jcat.2000.3018
Stuve EM, Madix RJ (1985) Use of the pi-sigma. Parameter for characterization of rehybridization upon adsorption on metal surfaces. J Phys Chem 89(15):3183–3185. doi:10.1021/j100261a001
Neurock M, Mei D (2002) Effects of alloying Pd and Au on the hydrogenation of ethylene: an Ab initio based dynamic Monte Carlo study. Top Catal 20(1):5–23
Aas N, Bowker M (1993) Adsorption and autocatalytic decomposition of acetic acid on Pd(110). J Chem Soc-Faraday Trans 89(8):1249–1255. doi:10.1039/ft9938901249
Bowker M, Morgan C, Couves J (2004) Acetic acid adsorption and decomposition on Pd(110). Surf Sci 555(1–3):145–156. doi:10.1016/j.susc.2003.12.040
Li Z, Gao F, Tysoe WT (2008) Surface chemistry of acetic acid on clean and oxygen-covered Pd(100). Surf Sci 602(2):416–423. doi:10.1016/j.susc.2007.10.045
Owens TG, Jones TE, Noakes TCQ, Bailey P, Baddeley CJ (2006) The effects of gold and Co-adsorbed carbon on the adsorption and thermal decomposition of acetic acid on Pd{111}. J Phys Chem B 110(42):21152–21160. doi:10.1021/jp062988a
Li Z, Calaza F, Gao F, Tysoe WT (2007) The adsorption of acetic acid on Au/Pd(111) alloy surfaces. Surf Sci 601(5):1351–1357. doi:10.1016/j.susc.2006.12.079
Li Z, Tysoe WT (2012) The adsorption of acetic acid on clean and oxygen-covered Au/Pd(100) alloy surfaces. Surf Sci 604(23–24):1934–1941. doi:10.1016/j.susc.2012.08.001
Calaza F, Stacchiola D, Neurock M, Tysoe WT (2005) Structure and decomposition pathways of vinyl acetate on Pd(111). Surf Sci 598(1–3):263–275. doi:10.1016/j.susc.2005.09.028
Li Z, Calaza F, Plaisance C, Neurock M, Tysoe WT (2009) Structure and decomposition pathways of vinyl acetate on clean and oxygen-covered Pd(100). J Phys Chem C 113(3):971–978. doi:10.1021/jp806729c
Morgan C, Bowker M (2009) The reaction of vinyl acetate with Pd(110) studied with TPD and molecular beams. Surf Sci 603(1):54–59. doi:10.1016/j.susc.2008.10.024
Calaza F, Li Z, Gao F, Boscoboinik J, Tysoe WT (2008) The adsorption and reaction of vinyl acetate on Au/Pd(111) alloy surfaces. Surf Sci 602(22):3523–3530. doi:10.1016/j.susc.2008.09.028
Rivalta I, Mazzone G, Russo N, Sicilia E (2009) Adsorption of ethylene, vinyl, acetic acid, and acetate species on PdAu(111) and PdAu(100) surface alloys: a cluster model study. J Chem Theory Comput 5(5):1350–1360. doi:10.1021/ct9000137
Li Z, Calaza F, Tysoe WT (2012) The adsorption and reaction of vinyl acetate on Au/Pd(100) alloy surfaces. Surf Sci 606(13–14):1113–1119. doi:10.1016/j.susc.2012.03.011
Soto-Verdugo V, Metiu H (2007) Segregation at the surface of an Au/Pd alloy exposed to CO. Surf Sci 601(23):5332–5339. doi:10.1016/j.susc.2007.08.022
Calaza F, Li ZJ, Tysoe WT (2011) Reaction between ethylene and acetate species on clean and oxygen-covered Pd(100): implications for the vinyl acetate monomer formation pathway. Catal Lett 141(2):266–270. doi:10.1007/s10562-010-0488-8
Calaza F, Stacchiola D, Neurock M, Tysoe WT (2010) Kinetic parameters for the elementary steps in the palladium-catalyzed synthesis of vinyl acetate. Catal Lett 138(3–4):135–142. doi:10.1007/s10562-010-0386-0
Redhead PA (1962) Thermal desorption of gases. Vacuum 12:9
Han YF, Kumar D, Sivadinarayana C, Goodman DW (2004) Kinetics of ethylene combustion in the synthesis of vinyl acetate over a Pd/SiO2 catalyst. J Catal 224(1):60–68. doi:10.1016/j.jcat.2004.02.028
Kragten DD, van Santen RA, Crawford MK, Provine WD, Lerou JJ (1999) A spectroscopic study of the homogeneous catalytic conversion of ethylene to vinyl acetate by palladium acetate. Inorg Chem 38(2):331–339. doi:10.1021/ic980399g
Nakamura S, Yasui T (1970) The mechanism of the palladium-catalyzed synthesis of vinyl acetate from ethylene in a heterogeneous gas reaction. J Catal 17(3):366–374. doi:10.1016/0021-9517(70)90113-2
Crathorne EA, Macgowan D, Morris SR, Rawlinson AP (1994) Application of isotopic transient kinetics to vinyl acetate catalysis. J Catal 149(2):254–267. doi:10.1006/jcat.1994.1294
Acknowledgments
We gratefully acknowledge support of this work by the National Science Foundation under grant number CHE 1109377 and the U.S. Department of Energy, Division of Chemical Sciences, Office of Basic Energy Sciences, under Grant No. DE-FG02-92ER14289.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paper submitted for publication in a Special Issue of Topics in Catalysis devoted to D. Wayne Goodman.
Rights and permissions
About this article
Cite this article
Neurock, M., Tysoe, W.T. Mechanistic Insights in the Catalytic Synthesis of Vinyl Acetate on Palladium and Gold/Palladium Alloy Surfaces. Top Catal 56, 1314–1332 (2013). https://doi.org/10.1007/s11244-013-0153-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-013-0153-8