Abstract
Rhodium colloids have been prepared by chemical reduction of an aqueous solution of rhodium trichloride with different aliphatic alcohols (from methanol to pentanol) in the presence of polyvinylpyrrolidone (PVP) as the stabilizing agent. The obtained Rh(0) nanoparticles have been characterized structurally by means of X-ray powder diffraction, scanning electron microscopy and transmission electron microscopy. Catalytic activity of the synthesized Rh/PVP colloids has been evidenced in the Suzuki–Miyaura coupling reaction of phenylboronic acid with bromobenzaldehyde. The choice of the reducing agent and reduction conditions enabled to obtain Rh(0) colloids showing various mean nanoparticle diameters (ranging from 3.8 to 6.0 nm) and different nanoparticle morphologies. These two factors play the decisive role from point of view of catalytic activity of the presented systems. The most active Rh/PVP catalyst, prepared using ethanol as the reducing agent, have also been applied in hydrogenation of benzene to cyclohexane, showing very high activity in this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transition metal nanoparticles are intermediate species between single atoms and a bulk material. They might be obtained by a variety of physical or chemical methods. According to the literature [1], the four most common techniques are: metal vapor condensation, thermal or photochemical decomposition of metal complexes, metal salt reduction, and electrochemical synthesis. The most frequently employed method of nanoparticles preparation is transition metal salt or organometalic precursor reduction in the presence of stabilizing agents, such as polymers or surfactants, which prevent the formation of aggregated metal particles by providing a steric barrier [1–3]. The reducing agents commonly used in nanoparticles synthesis [3–5] are: hydrogen, hydrazine and aliphatic alcohols.
Catalytic systems based on metal nanoparticles are relatively simple. The essential parameter that influences their activity is a surface to volume ratio [1]. A high value of that factor is an indicator of better access of the substrates to the catalytic centers due to fact that a large percentage of metal atoms lie on the surface of the nanoparticle. However, their properties depend also on other factors, i.e. the shape, crystalline structure, organization, etc. It is important to remember that the surface atoms do not necessarily order themselves in the same way that those in the bulk do [6]. Presently, the most common objectives of the researchers are focused on the precise control of the mean size and the surface structure to get well-defined nanocatalysts showing reproducible activity and selectivity [2].
Rhodium is one of the rarest and the most costly metals. Nevertheless, it exhibits remarkable and often unique catalytic properties in comparison with other noble metals, most particularly in hydrogenation reaction [7–11]. Rhodium in form of nanoparticles, situated at the border between homogeneous and heterogeneous catalysis, is even more versatile [12–15].
In this paper we report rhodium nanocolloids preparation using aliphatic alcohols as the reducing agents [16–20] and polyvinylpyrrolidone (PVP) as the stabilizing polymer. The obtained nanoparticles have been characterized structurally using X-ray powder diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
Carbon–carbon bond-forming processes are known as versatile tools in organic synthesis [21–24]. Among them, a special place is occupied by the Suzuki–Miyaura reaction, which is an efficient method of asymmetric biaryls preparation from an aryl halide and boronic acid, with high tolerance to the presence of functional groups as the substituents [25, 26]. The catalytic activity of the synthesized Rh(0) nanoparticles was tested in the Suzuki–Miyaura cross-coupling, which is commonly known as a palladium-catalyzed process [27, 28]. However, the application of rhodium in such a reaction shows the great versatility of Rh(0) nanoparticles. Only few examples of rhodium-catalyzed Suzuki–Miyaura cross-couplings have been published [29–31]. The obtained Rh/PVP colloids have also proved their high activity in hydrogenation of benzene, which represents a more typical kind of reaction catalyzed by rhodium compounds.
2 Experimental
2.1 Materials
Rh(acac)(P(OPh3)2 was prepared according to the reported synthesis procedure [32]. All other analytical grade reagents were purchased and used as received without additional purification.
2.2 Characterizations
The XRD measurements were carried out with a DRON-1 diffractometer operating with the Cu-Kα radiation line. The average diameter of the supported rhodium nanoparticles was estimated from the X-ray (111) diffraction line broadening (measured at 2θ = 41.05° with a step size of 0.01°) by means of the Debye–Scherrer equation.
Transmission electron microscopy measurements were carried out using a FEI Tecnai G2 20 X-TWIN electron microscope operating at 200 kV. The nanoparticle sizes distributions were determined by counting the size of ~200 rhodium nanoparticles from several TEM images obtained from different places of the TEM grids. The size distribution plots were next fitted using Gauss curve approximation. The SEM images were acquired using a Hitachi S-3400N scanning electron microscope.
Catalytic reaction products were identified and analyzed with a HP 5890 Series II gas chromatograph connected to HP 5971A mass selective detector. Separation was achieved on a capillary HP-5 column coated with a diphenyl (5 %) dimethylsiloxane (95 %) copolymer film. Helium was used as the carrier gas. NMR spectra were recorded at room temperature on a Bruker Avance 500 MHz spectrometer.
2.3 Rh/PVP Colloids Preparation
Polyvinylpyrrolidone 0.475 g (average molecular weight 40,000 g/mol) was added to 225 mL of alcohol (methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol or 1-pentanol) and intensively stirred until the polymer was totally dissolved. Next, 25 mL of an aqueous solution of RhCl3·3H2O containing 1 mg Rh in 1 mL was added. The solution was refluxed for 1.5 h. While heating, the mixture changed in color from light red to dark brown. Finally, the obtained colloidal suspension was dried completely in a rotary vacuum evaporator yielding a film of PVP stabilized Rh(0) colloid containing 5 % of Rh.
2.4 Suzuki–Miyaura Reaction
The catalytic test reactions were carried out in a 40 mL Schlenk tube with magnetic stirring. First, the solid reagents: phenylboronic acid 0.182 g (1.5 mmol) and potassium hydroxide 0.084 g (1.5 mmol) were introduced, then the liquids: mixture of solvents consisting of 1,4-dioxane 8 mL and water 2 mL, n-dodecane 0.100 mL (the internal standard) and 2-bromobenzaldehyde 0.118 mL or 4-bromobenzaldehyde 0.185 g (1.0 mmol). Finally, the Rh/PVP catalyst 0.04 g (2.0 × 10−2 mmol Rh) was added. The reactor was sealed under a nitrogen atmosphere and placed in a thermostated oil bath. The reactions were carried out at 80 °C for 24 h. Afterwards, the reactor was cooled down to the ambient temperature and the organic components were extracted by intensive shaking for 5 min with two portions of 5 mL of n-hexane. After the extraction, a small amount of water was added to facilitate separation of the liquid phases. A colorless n-hexane phase was transferred to a 10 mL calibrated flask and analyzed with GC–MS.
2.5 Hydrogenation of Benzene
Hydrogenation reactions were carried out in a 100 mL stainless steel autoclave equipped with a manometer, thermostat, magnetic stirrer and gas inlet/outlet system. In a typical experiment 2 mL of benzene (22 mmol) were placed in the reactor. In case of experiments carried out under biphasic liquid/liquid conditions 2 mL of water were added. Finally, the catalyst was introduced (1.1 × 10−2–1.1 × 10−3 mmol Rh), the autoclave was flushed with hydrogen and then loaded to the pressure of 20 bar. The reaction temperature was maintained at 80 °C for 4 h. Afterwards, the reactor was cooled down to the ambient temperature and the organic phase was analyzed with 1H NMR using CDCl3 as a solvent.
3 Results and Discussion
3.1 Structure of Rh/PVP Colloids
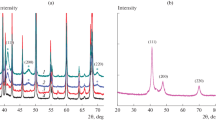
Rhodium nanocolloids were prepared by chemical reduction of an aqueous solution of rhodium trichloride with different aliphatic alcohols in the presence of polyvinylpyrrolidone as the stabilizing agent. The structure and phase composition of the obtained compounds were determined by X-ray powder diffraction. A typical diffractogram of a Rh/PVP colloid reveals characteristic background line originating from the protective polymer and several well-defined peaks (Fig. 1), which are attributed to Rh(0) crystallizing in Fm3m space group (JCPDS card number 5-685). Broadening of the diffraction lines confirms nanocrystalline nature of the synthesized colloids. The average rhodium particle size estimated from the half-width of the X-ray (111) for the system obtained by reduction with ethanol (Fig. 1) is 3.6 nm.
Scanning electron microscopy measurements were performed to visualize macroscopic surface structure of the Rh/PVP colloids. The obtained SEM micrographs (Fig. 2) show that the colloids are composed of relatively large lamellar blocks of polymer (several tens micrometers long). Only few fragments smaller than 10 μm might be noticed.
More accurate information about Rh/PVP colloids structure was obtained with transmission electron microscopy. The colloid synthesized using ethanol as the reducing agent is composed of small Rh(0) nanoparticles that are uniformly dispersed in the polymer matrix without any aggregation (Fig. 3a). The size distribution of the particles is symmetric with a narrow half-width of 1.2 nm and the centre at located at 3.5 nm.
Quite different results were observed in case of the Rh/PVP prepared by reduction with 2-butanol. The size distribution of the Rh(0) nanoparticles is still symmetric, however it is slightly broader and its centre is shifted towards larger particles: half-width at 1.6 nm, maximum located at 4.6 nm (Fig. 3b). Although the two colloids are characterized by quite similar size distributions, the most significant in this case are their morphological differences. Nanoparticles obtained by reduction with 2-butanol are quite irregularly shaped and most likely polycrystalline (shown on the magnified fragment of the micrograph). Very similar structure of the particles was also observed in PVP-stabilized Rh(0) colloids synthesized from Rh(I) complexes reduced with vanadocene V(C2H5)2 [33].
The observation that stronger reducing agents lead to small nanoparticles in a rapid reaction, whereas weaker reducing agents produce larger particles is in agreement with our earlier studies of PVP-protected Pd(0) colloids [34, 35]. The growth of the larger polycrystalline particles, in case of application of 2-butanol as the reducing agent, might be also explained from the thermodynamical point of view. At the early stage of reduction small and larger nanocrystals are formed. However, only those which exceed a certain critical size are thermodynamically stable. Smaller particles or nuclei either dissolve or attach to other nanocrystals, consequently forming bigger species in the coalescence process. The critical size is directly proportional to the surface energy [36], thus it varies with the choice of different solvents and reducing agents.
3.2 Suzuki–Miyaura Reaction
In order to compare the activity of rhodium nanoparticles, several Rh/PVP colloids have been prepared using different aliphatic alcohols (from methanol to pentanol) as the reducing agents. The Suzuki–Miyaura cross-coupling of phenylboronic acid with 2-bromobenzaldehyde or 4-bromobenzaldehyde, chosen as representative substrates of different steric hindrance (Fig. 4), was carried out in a mixture of 1,4-dioxane with water (4:1) at 80 °C using KOH as the base (Table 1). Such reaction conditions had been chosen as the optimal after a series of initial experiments in various solvents and with addition of different bases.
The obtained catalysts are characterized by different average crystallite size (estimated using XRD). Generally, the longer is the aliphatic chain of the alcohol used in the colloid synthesis the larger Rh(0) particles are obtained. The mean size of the Rh(0) particles increased with alkyl chain length (Fig. 3). Undoubtedly, as it is often postulated in literature [37–39], the nanoparticle size is one of the key factors affecting catalytic activity of transition metal colloids. It may be evidently seen (Table 1) that the yield of the product in the Suzuki–Miyaura reaction is dependent on the particle size. The most active are the colloids prepared using methanol and ethanol which are the smallest ones. Remarkably lower activity was noted when Rh(0) particles obtained by reduction with 1-butanol or 2-butanol were applied. However, the XRD-estimated average crystallite size does not cover full structural information and consequently it is not the only factor affecting catalytic activity [35, 40–42]. As it has already been shown before (Fig. 3), rhodium colloids obtained using heavier aliphatic alcohols may contain many significantly aggregated particles. The surface to volume ratio of such species is much lower than it might be suggested by the average crystallite size (measured by XRD) thus their activity is also considerably lower.
For the reuse studies (Table 1; runs 2–4), the organic reaction products were extracted with n-hexane and then a new portion of the substrates (phenylboronic acid, 2-bromobenzaldehyde or 4-bromobenzaldehyde) and the base were added to the reaction medium (1,4-dioxane with water) containing dissolved Rh/PVP colloid. The Rh(0) nanoparticles obtained by reduction with ethanol preserved their catalytic activity while recycling but a slight decrease in yield was observed. Indeed, in the fourth reaction run, carried out with 2-bromobenzaldehyde or 4-bromobenzaldehyde as the aryl halide, the yield of the product was 30 and 42 %, respectively against 42 and 62 % in the first run.
The observed deactivation of the catalyst in successive runs might be explained by leaching of rhodium in form of soluble complexes. The widely used test to identify the nature of the catalyst is mercury poisoning [43, 44]. The inhibition of catalysis by Hg(0), which amalgamate the metal catalyst or adsorbs on its surface, is an evidence for heterogeneous catalysis as well as the presence of Rh(0) species in the system. In our case, the addition of an excess of mercury (500 equivalents) before the reaction causes very significant, however not complete, suppression of catalytic activity. The yield of the product: 2-phenylbenzaldehyde or 4-phenylbenzaldehyde is 10 and 14 %, respectively. These results show that, besides heterogeneous Rh(0) nanoparticles, also monomolecular species, most probably originating from Rh(0) colloids, could be involved in the catalytic process. These species might leach during extraction of organic products, thus the activity of the catalysts is lowered in subsequent runs.
3.3 Hydrogenation of Benzene
Hydrogenation reactions are the most often cited processes for which rhodium-based catalyst are developed. In our early studies [8] we found that rhodium(I) acetyloacetonato-bis-triphenylphosphite complex, prepared by reaction of Rh(acac)(CO)2 with P(OPh)3 [32], may catalyze hydrogenation of benzene, yielding a few percent of cyclohexane as the only reaction product [8]. However, nowadays we have noticed that the starting complex Rh(acac)(CO)2 without phosphorus ligands is even more active and yields 33 % of the product in the same reaction (Table 2). Such a big difference might be explained by the fact that the Rh(I) complex is reduced under reaction conditions to Rh(0) nanoparticles, which are the active form of the catalyst. The in situ reduction of transition metal complexes to metal particles is commonly observed [45]. The reduction was also confirmed in our case by the TEM analysis of the black deposit formed in the hydrogenation reaction catalyzed with Rh(acac)(CO)2. The TEM images evidenced the presence of quite large Rh(0) crystals (50–100 nm), however smaller particles (10–15 nm) could also be found. High resolution TEM micrographs confirmed atomic arrangement characteristic for the FCC structure of Rh(0) with the (111) interplanar distance of 0.219 nm. Conversely, the phosphite-containing complex Rh(acac)(P(OPh)3)2 exhibits relatively high stability of the coordination sphere, thus is more difficult to reduce to Rh(0) and consequently shows much lower activity in the hydrogenation of benzene.
These previous results stimulated us to apply our Rh/PVP catalyst in catalytic hydrogenation of benzene to cyclohexane. For the tests we chose the Rh(0) colloid prepared using ethanol as the reducing agent, which had previously shown the highest activity in the Suzuki–Miyaura coupling of phenylboronic acid with bromobenzaldehyde.
Surprisingly, in the reaction carried out under conditions identical to those applied in case of the Rh(I) complexes no cyclohexane was formed. This might be justified by the fact that the protective polymer (PVP) is completely insoluble in benzene, which was both the substrate and the reaction media. For this reason we decided to perform further experiments in biphasic liquid/liquid system. Among different biphasic approaches, water appears to be the most desired green reaction medium and PVP dissolves in water very well. In the reaction system containing 2 mL of water 100 % of benzene was converted to cyclohexane after 4 h (Table 2). Even when the catalyst to hydrocarbon ration was lowered 5 or 10 times, the system remained still very active yielding 48 and 22 % of the product, respectively.
The application of a biphasic liquid/liquid system enables easy separation of the organic layer containing the substrate and the product from the aqueous phase containing Rh(0) nanoparticles. Thus the catalyst might be recycled several times in subsequent reaction runs. Our system remained active in five consecutive cycles, gradually losing its activity (Table 2). In our opinion, the observed deactivation should be attributed to morphological changes of the Rh/PVP catalyst. TEM micrograph of the catalyst recovered after first reaction run clearly reveals considerable aggregation of the nanoparticles (Fig. 5a). Quite large species had been formed (more than 50 nm in diameter) that are probably catalytically inactive. Nevertheless there are still numerous particles that did not suffer aggregation and are quite well dispersed in the reaction medium (Fig. 5b). On the other hand, physical processing of the catalyst, e.g. losing some part of the rhodium-containing aqueous phase after each reaction run might be also considered as a factor responsible for reducing activity of the system in consecutive reactions. We did not detect any leaching of rhodium to the organic phase; the ICP analyses did not reveal any traces of the metal.
4 Conclusions
Several PVP-stabilized Rh(0) nanocolloids have been prepared by chemical reduction of an aqueous solution of rhodium trichloride with different aliphatic alcohols. The morphology and size distribution of rhodium nanoparticles are dependent on the alcohol used. Methanol and ethanol produce rather small Rh(0) particles while heavier alcohols generate bigger and aggregated species. The size and the morphology of the nanoparticles play the important role from the point of view of catalytic activity of the obtained colloids. The highest product yield in Suzuki–Miyaura coupling of phenylboronic acid with bromobenzaldehyde have been observed for Rh/PVP catalyst prepared using ethanol as the reducing agent. The catalyst could be recycled several times.
The same catalyst has also shown very high activity in hydrogenation of benzene to cyclohexane. The main advantage of our system is a very high activity, when compared to monomolecular rhodium complexes [7–10]. The application of biphasic conditions enables easy separation of the organic products from the catalyst. The recyclability of the described system might be additionally improved by using other protecting agents than PVP, for instance functionalized polymers [15, 42], or by supporting Rh(0) nanoparticles on oxide supports like alumina [40, 41] or silica [12].
References
Aiken JD, Finke RG (1999) J Mol Catal A 145:1–44
Cushing BL, Kolescnichenko VL, O’Connor CJ (2004) Chem Rev 104:3893–3946
Roucoux A, Schulz J, Patin H (2002) Chem Rev 102:3757–3778
Lewis LN (1993) Chem Rev 93:2693–2730
Wang X, Zhuang J, Peng Q, Li Y (2005) Nature 437:121–124
Pool R (1990) Science 248:1186–1188
Hirai H, Ohtaki M, Komiyama M (1978) Chem Lett 1:149–152
Pieta D, Trzeciak AM, Ziółkowski JJ (1983) J Mol Catal 18:193–195
Duan Z, Hampden-Smith MJ (1992) Chem Matter 4:1146–1148
Rossen K (2001) Angew Chem Int Ed 40:4611–4613
Pellegatta JL, Blandy C, Colliere V, Choukroun R, Chaudret B, Cheng P, Philippot K (2002) J Mol Catal A 178:55–61
Barthe L, Denicourt-Nowicki A, Roucoux A, Philippot K, Chaudret B, Hemati M (2009) Catal Commun 10:1235–1239
Thomas JM (2010) Chem Cat Chem 2:127–132
Yuan Y, Yan N, Dyson PJ (2012) ACS Catal 2:1057–1069
Noël S, Léger B, Herbois R, Ponchel A, Tilloy S, Wenz G, Monflier E (2012) Dalton Trans 41:13359–13363
Hirai H, Nakao Y, Toshima N, Adachi K (1976) Chem Lett 5:905–910
Hirai H, Nakao Y, Toshima N (1978) J Macromol Sci Chem A 12:1117–1141
Hirai H (1979) J Macromol Sci Chem A 13:633–649
Hirai H, Nakao Y, Toshima N (1979) J Macromol Sci Chem A 13:727–750
Komiyama M, Hirai H (1983) Bull Chem Soc Jpn 56:2833–2834
Diederich F, Stang PJ (1997) Metal-catalyzed cross-coupling reactions. VCH, Weinheim
Beletskaya IP, Cheprakov AV (2000) Chem Rev 100:3009–3066
Trzeciak AM, Ziółkowski JJ (2005) Coord Chem Rev 249:2308–2322
Trzeciak AM, Ziółkowski JJ (2007) Coord Chem Rev 251:1281–1293
Miyaura N, Yanagi T, Suzuki A (1981) Synth Commun 11:513–519
Miyaura N, Suzuki A (1995) Chem Rev 95:2457–2483
Tsuji J (2004) Palladium reagents and catalysts. Wiley, New York
Diederich F, Stang P (2008) Metal-catalyzed cross-coupling reactions. Wiley, New York
Ueura K, Satoh T, Miura M (2005) Org Lett 7:2229–2231
Wu J, Zhang L, Luo Y (2006) Tetrahedron Lett 47:6747–6750
Kantam ML, Roy S, Roy M, Sreedhar B, Choudary BM, De RL (2007) J Mol Catal A 273:26–31
Trzeciak AM, Ziółkowski JJ (1982) Inorg Chim Acta Lett 64:L267–L268
Choukroun R, de Caro D, Chaudret B, Lecante P, Snoeck E (2001) New J Chem 25:525–527
Gniewek A, Trzeciak AM, Ziółkowski JJ, Kępiński L, Wrzyszcz J, Tylus W (2005) J Catal 229:332–343
Gniewek A, Ziółkowski JJ, Trzeciak AM, Kępiński L (2006) J Catal 239:272–281
Hartman P (1973) Crystal growth: an introduction. American Elsevier, New York
Narayanan R, El-Sayed M (2003) J Phys Chem B 107:12416–12424
Narayanan R, El-Sayed M (2004) J Phys Chem B 108:8572–8580
Hu J, Liu Y (2005) Langmuir 21:2121–2123
Gniewek A, Ziółkowski JJ, Trzeciak AM, Zawadzki M, Grabowska H, Wrzyszcz J (2008) J Catal 254:121–130
Mieczyńska E, Gniewek A, Pryjomska-Ray I, Trzeciak AM, Grabowska H, Zawadzki M (2011) Appl Catal A 393:195–205
Borkowski T, Zawartka W, Pospiech P, Mizerska U, Trzeciak AM, Cypryk M, Tylus W (2011) J Catal 282:270–277
Whitesides GM, Hackett M, Brainard RL, Lavalleye J-PPM, Sowinski AF, Izumi AN, Moore SS, Brown DW, Sraudt EM (1985) Organometallics 4:1819–1830
Fernández F, Cordero B, Durand J, Muller G, Malbosc F, Kihn Y, Teuma E, Gómez M (2007) Dalton Trans: 5572–5581
Widegreen JA, Finke RG (2003) J Mol Catal A 198:317–341
Acknowledgments
This study was partially supported by University of Wrocław, Faculty of Chemistry with grant No. 1489/M/WCH/11. The financial support is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gniewek, A., Trzeciak, A.M. Rh(0) Nanoparticles: Synthesis, Structure and Catalytic Application in Suzuki–Miyaura Reaction and Hydrogenation of Benzene. Top Catal 56, 1239–1245 (2013). https://doi.org/10.1007/s11244-013-0090-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-013-0090-6