Abstract
Ruthenacycles, which are easily prepared in a single step by reaction between enantiopure aromatic amines and [Ru(arene)Cl2]2 in the presence of NaOH and KPF6, are very good asymmetric transfer hydrogenation catalysts. A range of aromatic ketones were reduced using isopropanol in good yields with ee’s up to 98%. Iridacycles, which are prepared in similar fashion from [IrCp*Cl2]2 are excellent catalysts for the racemisation of secondary alcohols and chlorohydrins at room temperature. This allowed the development of a new dynamic kinetic resolution of chlorohydrins to the enantiopure epoxides in up to 90% yield and 98% enantiomeric excess (ee) using a mutant of the enzyme Haloalcohol dehalogenase C and an iridacycle as racemisation catalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Asymmetric hydrogenation is arguably one of the most important methods for the catalytic synthesis of enantiopure compounds [1, 2]. We have previously analysed that the relative low use of this technology in the production of pharmaceuticals, agrochemicals and flavour and fragrance products is due among others to the limited development time available and the high costs of the catalysts [3]. To counter this problem, we have developed a new class of low cost ligands for asymmetric hydrogenation that can be prepared in just two steps. Binol-based monodentate phosphoramidites can be synthesised in a mere 2 steps, making them not only highly cost-effective but also readily prepared in a short period of time even in kg amounts [4]. In addition, their easy preparation allows the parallel synthesis of 96 ligands simultaneously in high throughput equipment, enabling the synthesis and testing of these ligands within 2 days [5]. This approach has proven its usefulness in practice and has led to a ton-scale asymmetric hydrogenation process based on a rhodium phosphoramidite/triphenylphosphine catalyst [6].
We wanted to develop a similar approach to asymmetric transfer hydrogenation. This technology has the advantage that it can be easily applied on large scale without the need for high pressure equipment. The ligand types are even more diverse than those applied in asymmetric hydrogenation [7–9]. And although transfer hydrogenation catalysts are quite sensitive towards oxygen, they seem to be less sensitive to other impurities stemming from starting materials or solvents than the classical rhodium-, iridium- and ruthenium-based asymmetric hydrogenation catalysts. The first real breakthrough in this field stems from the work of Noyori and co-workers who developed catalysts that can be assembled in situ by reacting [Ru(arene)Cl2]2 with tosylated diamines, aminoalcohols or bis(aminophosphines) [10]. These catalysts led to high enantioselectivities in the asymmetric reduction of selected ketones and imines. Others have developed this field further and a great variety of ligands have been developed that include amino acid and peptide derivatives, aminoethers and aminothioethers [7–9]. The minimum requirement seems to be an amino group that carries at least one proton. These ligands are non-innocent as the proton on the amine participates in the transfer hydrogenation [11].
We were interested in developing a new class of asymmetric transfer hydrogenation catalysts based on a simple class of ligands that are modular and are easy to synthesise.
2 Experimental Section
Complexes 1–3 [12–14], 4a [15, 16], 4b [17], 6 [18], 7 [19], 8a–c [20] were prepared according to literature procedures.
2.1 Typical Procedure for the Catalytic Transfer Hydrogenation
The catalyst (10 μmol) was dissolved in 2-propanol (10 mL) under argon, and acetophenone (120 mg, 1 mmol) was added, followed by tBuOK (5.6 mg, 50 μmol). The reaction was periodically monitored by GC. Upon completion the solvent was evaporated, the crude product was dissolved in Et2O and filtered over silica gel using Et2O as eluent. The conversions and enantiomeric excess (ee) values were determined by GC using a chiral capillary column (Chiraldex β-PM, 50 m × 0.25 mm × 0.25 μm).
2.2 Catalytic Racemisation of Alcohols
In a flame-dried Schlenk flask under an atmosphere of nitrogen, 37.5 μmol of catalyst and 41.2 μmol of KOtBu were dissolved in 2.4 mL of freshly distilled toluene, after which 0.75 mmol of chiral alcohol was added. The reaction was monitored by periodically taking 0.1 mL aliquots from the mixture, filtering them over silica gel (eluent: Et2O) and analyzing the resulting samples by chiral GC (Chirasil-Dex CB column (25 m × 0.25 mm × 0.25 μm), gas vector: helium, flow: 1 mL/min. Injector: 250 °C. Program: 100 °C for 3 min, 120 °C (15 °C/min) for 15 min, 140 °C (15 °C/min) for 15 min, 100 °C (15 °C/min) or chiral HPLC (OD-H column, hexane/isopropanol (95/5)).
2.3 General Method for the DKR of β-Haloalcohols
A 50 mL two-necked flask was charged with 10 mL of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (50 mM, pH 8.0), a solution of 200 μmol of substrate in 0.5 mL of distilled DMSO, 35 mg of bovine serum albumin (BSA), and about 6 UFootnote 1 of haloalcohol dehalogenase C (C153S, W 249F).Footnote 2 Then, a solution of 8b (6.3 mg, 10 μmol, 5 mol%) and KOtBu (1.2 mg, 11 μmol) in 3 mL of freshly distilled toluene was added to the solution over 6 h using a syringe pump. To analyze the composition of the reaction mixture, 0.1 mL samples were taken from the organic layer, filtered over silica (eluent: Et2O), concentrated in vacuo, redissolved in heptane/IPA and analyzed by chiral HPLC or GC. In those cases where the products were isolated, the reaction mixture was extracted with EtOAc, dried over MgSO4, filtered, and the filtrate concentrated under reduced pressure. The resulting crude products were purified by column chromatography over silica gel, using mixtures of heptane and EtOAc as eluent.
2.4 Ruthenacycles as Transfer Hydrogenation Catalysts
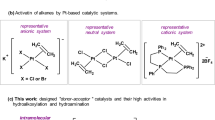
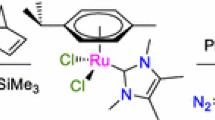
As part of a CH-activation project, we were investigating ruthenacycle 1a, which is capable of inserting olefins into the metal–carbon bond [22, 23].Footnote 3 This ruthenacycle, which is surprisingly stable is very easily synthesised from [Ru(arene)Cl2]2 according to Scheme 1 [19]. A simple flash column over alumina gave essentially pure material. These ruthenacycles are chiral at ruthenium, however, they are not configurationally stable. We assume that easy racemisation occurs by a dissociation/association of the acetonitrile ligand. The diastereomeric ratio (dr) of the ruthenacycle depends of course on the ligands. In the case of 1a the dr was 74:26 [19].
Use of ruthenacycle 1a as catalyst (S:C = 100) in the asymmetric transfer hydrogenation of acetophenone, using iso-propanol as reductant and solvent and KOtBu as activator led to a 48% yield of 1-phenylethanol after 2 h with a product ee of 10% (Table 1, entry 1) [25].

As this catalyst possesses no proton on the amino ligand, we must assume a mechanism different than the one proposed by Noyori. Encouraged by this first result we decided to synthesise the ruthenacycle 1b, which contains an NH2-group. Indeed, not only was the transfer hydrogenation rate much higher, also the ee of the product alcohol improved to 38% (Entry 2). As we wanted to screen a broad range of chiral aromatic primary and secondary amines we decided to use a High Throughput Experimentation (HTE) approach. Indeed, we were able to automate both the synthesis of the ruthenacycles as well as their screening in the transfer hydrogenation using a Lizzy liquid dispensing robot that is positioned in a glovebox [25]. After completion of the ruthenacycle synthesis the solvent acetonitrile, which is an inhibitor for the transfer hydrogenation was removed by blowing nitrogen through the vials; iso-propanol was added, followed by the substrate acetophenone and finally a solution of KOtBu (1.5 equiv to Ru) to activate the catalyst. In this way we screened a small library of nine amines, which led to the finding that in fact secondary aromatic amines are superior ligands. Three catalysts that gave very good results were the ruthenacycles 1c, 2 and 3 (Fig. 1). These were resynthesised and the good results were confirmed with the purified compounds. Use of catalyst 1c led to formation of the alcohol in virtually quantitative yield in just half an hour yielding the product in 76% ee (Entry 3). Lowering the temperature to 0 °C improved the ee further to 85%, although the rate slowed down somewhat (Entry 4). A very good ee of 89% was also obtained with catalyst 2 based on 2,5-diphenylpyrrolidine (Entry 5). Catalyst 3 based on 1-(2-naphthyl)-ethylamine turned out to be very fast: even after 10 min the alcohol was obtained in 90% yield (TOF = 540 h−1) although the ee was only a mediocre 57% (Entry 6). We next screened a range of aromatic substrates using the best two catalysts 1c and 2 which led to some interesting findings (Table 2).

It is clear from the results in Table 2 (Entry 2) that aromatic ketones containing electron withdrawing groups such as B are hydrogenated very fast (TOF > 500 h−1), but with low enantioselectivity. Another interesting finding is the fact that branching in the alpha position on the aliphatic side of the ketone leads to much improved enantioselectivities, particularly with catalyst 1c (Entries 3–5). Tetralone was reduced with excellent enantioselectivity by catalyst 2 (Entry 6). Both catalysts were able to reduce 4-acetyl-styrene without reducing the C–C double bond as well as 2-acetylfuran with very good enantioselectivity (Entries 7, 8).
2.5 Ruthenacycles and Iridacycles as Alcohol Racemisation Catalysts
In all of the above reductions it is observed that the enantioselectivity of the products decreases somewhat over time during the reaction. This is caused by the reversibility of the transfer hydrogenation. The enantiomer that is formed in excess is also the one that is more easily oxidized in the Oppenauer oxidation. Although this is a nuisance in the asymmetric transfer hydrogenation, this racemising capacity can actually be put to good use. The most well-known use is in the dynamic kinetic resolution (DKR) of alcohols as developed by Williams [26] and Bäckvall [27]. In this reaction only one of the enantiomers of a racemic alcohol is acylated, catalysed by a lipase enzyme (Scheme 2). The other enantiomer that remains unreacted is continuously racemised, thus leading to a 100% yield of the acylated alcohol. Bäckvall and co-workers initially used the Shvo catalyst 4a (Fig. 2) for the racemisation reaction, but later developed monomeric equivalents such as 5 that were even more active as racemisation catalyst [28]. This DKR has been further developed by Verzijl and co-workers leading to its application in a large-scale process at DSM [29]. Bäckvall and co-workers have shown that it is also possible to subject chlorohydrins to the same conditions to obtain enantiopure acylated chlorohydrins (Scheme 3) [30]. These compounds can then be treated with base to obtain the enantiopure epoxides. We were interested to develop a new DKR of chlorohydrins directly to the enantiopure epoxides by using a haloalcohol dehalogenase instead of a lipase.
DKR approach to enantiopure epoxides from Bäckvall [29]
The use of haloalcohol dehalogenase for the kinetic resolution of halohydrins has been largely developed by Janssen and co-workers (Scheme 4) [31–34]. The enzyme also catalyses the reverse reaction between epoxides and small anionic nucleophiles such as azide, cyanide or nitrite.
Thus, if a catalyst can be found that can racemise chlorohydrins a direct DKR becomes possible. However, unlike lipases, haloalcohol dehalogenases need to be dissolved in water for activity. In addition, these enzymes are not heat stable. This puts some extra restrictions on the racemisation catalyst: it should be able to function in the presence of water at moderate temperatures. Thus we screened a number of known catalysts in the racemisation of both phenylethanol (a) and 2-chloro-1-phenylethanol (b) (Table 3). Both catalysts 4a and 4b that were developed by Shvo are capable of racemising a as well as b, however, elevated temperatures are needed resulting in formation of ketone as side product. It is clear that this high temperature is not compatible with the enzyme. Much better results were obtained with catalyst 6 developed by Park and co-workers which was capable of full racemisation of both alcohols within half an hour at room temperature [19]. Unfortunately, the catalyst failed completely under aqueous conditions. Ruthenacycle 7 as expected was an excellent racemisation catalyst for a at room temperature and even functioned under aqueous conditions, albeit somewhat slower. However, the catalyst was incapable of racemising chlorohydrin b. We surmised that this could be due to catalyst inhibition caused by the chloroketone, which is a strong alkylating agent. Indeed upon addition of phenacyl chloride to the racemisation reaction of a, catalysed by 7, the reaction immediately stalled.

Encouraged by the high reactivity of the ruthenacycle 7 on a, we decided to test the iridacycles 8a–c. These compounds are made in similar fashion as the ruthenacycles starting from [Ir(Cp*)Cl2]2¸by reaction with the appropriate amine, NaOH and KPF6 in MeCN. They were isolated in pure form in good yields after a flash column over alumina. The catalysts are activated by treatment with KOtBu. We found a larger difference in reactivity dependent on the substitution pattern on the nitrogen. In this case, the catalyst based on the primary amine 8a is not better than the dimethyl analogue 8c as would be expected, but hardly showed any reactivity at room temperature (Fig. 3a). The catalyst based on the secondary amine clearly outperformed both catalysts and led to virtually complete racemisation after 24 h at room temperature. Surprisingly, racemisation of the chlorohydrin b proceeded much faster (Fig. 3b). Here catalysts 8a and 8c both showed good performance, but catalyst 8b led to very fast racemisation. Full racemisation was observed within 20 min. Better still, this catalyst also functions in aqueous/organic mixtures.
2.6 Dynamic Kinetic Resolution of Chlorohydrins
Having solved the problem of a finding a suitable racemisation catalyst we started working on the dynamic kinetic resolution of the chlorohydrins. First experiments were not encouraging as the enzyme and the racemisation catalyst somehow seemed to inhibit each other. We were able to solve these problems in the following way. The substrate chlorohydrin was dissolved in a little bit of DMSO and added to the enzyme solution in HEPES buffer. To protect the enzyme, bovine serum albumin (BSA) was added. The racemisation catalyst 8b was slowly added over a 6 h period as a toluene solution, thus mitigating its rapid deactivation. In view of its high activity this was sufficient to obtain a good conversion. Indeed after 16 h a 90% conversion of b was observed and the remaining 5% of chlorohydrin had an ee of 75% showing that the racemisation catalyst was still active (Scheme 5) [35]. The product epoxide was obtained in 98% ee. The protective action of BSA presumably is based on its preferential location at the aqueous/organic interphase, thus effectively preventing contact between the two catalysts. Having thus established the conditions for the successful DKR of aromatic chlorohydrins we next tested the scope of this reaction (Table 4).

In general, aromatic chlorohydrins with substituents in the 3- and 4-positions were efficiently converted (Table 4, entries 1–3, 5–7). However, ortho-substituents are not well tolerated by the enzyme and in this case the reaction is rather slow (Entry 4).
The attempted DKR of chloromethyl cyclohexyl ketone only proceeded in 50% yield and indeed the remaining chlorohydrin had a high ee, suggesting that this particular substrate is not efficiently racemised. However, less-hindered aliphatic alcohols such as 2-butanol and 2-hexanol could be efficiently racemised using catalyst 8b.
3 Conclusions
In conclusion, we have shown that ruthenacycles, prepared in a single step from enantiopure aromatic amines by reaction with [Ru(benzene)Cl2]2 in the presence of NaOH and KPF6, are efficient asymmetric transfer hydrogenation catalysts. Ruthenacycles are also efficient racemisation catalysts for secondary alcohols at room temperature, although they could not racemise chlorohydrins as the corresponding chloroketone turned out to be a catalyst inhibitor. Iridacycles prepared in a similar manner from the aromatic amines and [IrCp*Cl2]2 were not inhibited and also functioned well in an aqueous environment. This allowed the dynamic kinetic resolution of aromatic chlorohydrins to the enantiopure epoxides in very good yields by using a combination of iridacycle 8b and Haloalcohol dehalogenase C, which had been mutated in two positions.
References
de Vries JG, Elsevier CJ (eds) (2007) The handbook of homogenous hydrogenation, vols 1–3. Wiley-VCH, Weinheim
Special Issue on hydrogenation and transfer hydrogenation (2007) Acc Chem Res 12:1237–1419
de Vries JG, de Vries AHM (2003) Eur J Org Chem 799
Minnaard AJ, Feringa BL, Lefort L, de Vries JG (2007) Acc Chem Res 40:1267
Lefort L, Boogers JAF, de Vries AHM, de Vries JG (2004) Org Lett 6:1733
Boogers JAF, Felfer U, Kotthaus M, Lefort L, Steinbauer G, de Vries AHM, de Vries JG (2007) Org Proc Res Dev 11:585
Ikariya T, Blacker AJ (2007) Acc Chem Res 40:1300
Blacker AJ (2007) In: de Vries JG, Elsevier CJ (eds) The handbook of homogenous hydrogenation. Wiley VCH, Weinheim, p 1215
Gladiali S, Alberico E (2006) Chem Soc Rev 35:226
Noyori R, Hashiguchi S (1997) Acc Chem Res 30:97
Haack K-J, Hashiguchi S, Fujii A, Ikariya T, Noyori R (1997) Angew Chem Int Ed Engl 36:285
Sortais JB, Pannetier N, Holuigue A, Barloy L, Sirlin C, Pfeffer M, Kyritsakas N (2007) Organometallics 26:1856
Sortais JB, Pannetier N, Clement N, Barloy L, Sirlin C, Pfeffer M, Kyritsakas N (2007) Organometallics 26:1868–1874
Pannetier N, Sortais J-B, Dieng PS, Barloy L, Sirlin C, Pfeffer M (2008) Organometallics 27:5852
Blum Y, Shvo Y (1984) Isr J Chem 24:144
Blum Y, Czarkle D, Rahamim Y, Shvo Y (1985) Organometallics 4:1459
Shvo Y, Czarkie D, Rahamim Y (1986) J Am Chem Soc 108:7400
Choi JH, Kim YH, Nam SH, Shin ST, Kim M-J, Park J (2002) Angew Chem Int Ed 41:2373
Fernandez S, Pfeffer M, Ritleng V, Sirlin C (1999) Organometallics 18:2390
Jerphagnon T, Gayet AJA, Berthiol F, Ritleng V, Mršić N, Meetsma A, Pfeffer M, Minnaard AJ, Feringa BL, de Vries JG (2009) Chem Eur J 15:12780
Lutje Spelberg JH, Tang L, Van Gelder M, Kellogg RM, Janssen DB (2002) Tetrahedron Asymm 13:1083
Ritleng V, Pfeffer M, Sirlin C (2003) Organometallics 22:347
Ritleng V, Sutter JP, Pfeffer M, Sirlin C (2000) Chem Commun 129
Djukic J-P, Sortais J-B, Barloy L, Pfeffer M (2009) Eur J Inorg Chem 817
Sortais J-B, Ritleng V, Voelklin A, Holuigue A, Smail H, Barloy L, Sirlin C, Verzijl GKM, Boogers JAF, de Vries AHM, de Vries JG, Pfeffer M (2005) Org Lett 7:1247
Dinh PM, Howarth JA, Hudnott AR, Williams JMJ, Harris H (1996) Tetrahedron Lett 37:7623
Larsson ALE, Persson BA, Bäckvall J-E (1997) Angew Chem Int Ed Engl 36:1211
Martín-Matute B, Edin M, Bogár K, Kaynak FB, Bäckvall J-E (2005) J Am Chem Soc 127:8817
Verzijl GKM, de Vries JG, Broxterman QB (2005) Tetrahedron Asymm 16:1603
Träff A, Bogár K, Warner M, Bäckvall J-E (2008) Org Lett 10:4807
Janssen DB (2007) Adv Appl Microbiol 61:23
de Jong RM, Dijkstra BW (2003) Curr Opin Struct Biol 13:722
de Vries EJ, Janssen DB (2003) Curr Opin Biotechnol 14:414
Fetzner S, Lingens F (1994) Microbiol Rev 59:641
Haak RM, Berthiol F, Jerphagnon T, Gayet AJA, Tarabiono C, Postema CP, Ritleng V, Pfeffer M, Janssen DB, Minnaard AJ, Feringa BL, de Vries JG (2008) J Am Chem Soc 130:13508
Acknowledgements
Financial support from the Netherlands Organisation for Scientific Research (NWO-CW/STW), the Dutch Ministry of Economic Affairs, Royal DSM N.V., and N.V. Organon, administered through the IBOS program is gratefully acknowledged.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Jerphagnon, T., Haak, R., Berthiol, F. et al. Ruthenacycles and Iridacycles as Catalysts for Asymmetric Transfer Hydrogenation and Racemisation. Top Catal 53, 1002–1008 (2010). https://doi.org/10.1007/s11244-010-9569-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-010-9569-6