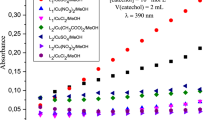
Abstract
Two novel cobalt(ii) complexes [Co(HL1)2](NO3)2.2.5H2O (1) and [Co(HL2)2](NO3)2 (2) (where HL1 = (E)-2-(1-(pyridin-2-yl)ethylidene)hydrazine-1-carboxamide and HL2 = (E)-2-(pyridin-2-ylmethylene)hydrazine-1-carboxamide) have been synthesized and structurally characterized by spectroscopic techniques and single-crystal diffraction analysis. The complexes are close comparable with metals exhibiting the expected distorted octahedral geometry being chelated by two semicarbazone ligands via NNO donor set. The catecholase-like activity of complexes 1 and 2 was evaluated by using 3,5-di-tert-butylcatecholas substrate. The results showed that both the complexes are effective catalysts with Kcat values of 762 and 562, respectively. Theoretical DFT study and Hirschfeld surface analyses were also carried out to reveal the nature of intermolecular contacts and to integrate experimental observations.
Graphical abstract
Two novel cobalt(ii) complexes [Co(HL1)2](NO3)2.2.5H2O (1) and [Co(HL2)2](NO3)2 (2) have been synthesized and structurally characterized by spectroscopic techniques and single-crystal diffraction analysis. The complexes are nearly akin with metals exhibiting the expected distorted octahedral geometry being chelated by two semicarbazone ligands via NNO donor set. The catecholase-like activity of complexes 1 and 2 was evaluated by using 3,5-DTBC as substrate. The result confirmed the formation of quinone or 3,5-DTBQ derivative and indicates that the complexes exhibit noticeable catalytic activity with Kcat values of 762 and 562, respectively. Theoretical DFT study and Hirschfeld surface analyses were also performed to reveal the nature of intermolecular contacts and to integrate experimental findings.












Similar content being viewed by others
References
Shaabani B, Khandar AA, Mahmoudi F, Balula SS, Cunha-Silva L (2013) Synthesis structure and electrochemistry behavior of a cobalt(III) compound with azide and methyl 2-pyridyl ketone semicarbazone ligands. J Mol Struct 1045:55–61. https://doi.org/10.1016/j.molstruc.2013.03.049
Meghdadi S, Amirnasar M, Mereiter K, Molaee H, Amiri A (2011) Synthesis, structure and electrochemistry of Co(III) complexes with an unsymmetrical Schiff base ligand derived from 2-aminobenzylamine and pyrrole-2-carboxaldehyde. Polyhedron 30:1651–1656. https://doi.org/10.1016/j.poly.2011.03.041
Fayed EA, Eldin RRE, Mehany ABM, Bayoumi AH, Ammar YA (2021) Isatin-Schiff’s base and chalcone hybrids as chemically apoptotic inducers and EGFR inhibitors, design, synthesis, anti-proliferative activities and in silico evaluation. J Mol Struct 1234:130159. https://doi.org/10.1016/j.molstruc.2021.130159
Gan KC, Sim KM, Lim TM, Teo KC (2020) Synthesis, Cytotoxic, antibacterial and free radical scavenging activities of new 1,2,4-Triazole schiff bases. Lett Org Chem 17:191–198. https://doi.org/10.2174/1570178616666190724114741
Hassan AS, Awad HM, Magd-El-Din AA, Hafez TS (2018) Synthesis and in vitro antitumor evaluation of novel Schiff bases. Med Chem Res 27:915–927. https://doi.org/10.1007/s00044-017-2113-5
Kordestani N, Rudbari HA, Fernandes AR et al (2021) Copper(II) complexes with tridentate halogen-substituted Schiff base ligands: synthesis, crystal structures and investigating the effect of halogenation, leaving groups and ligand flexibility on antiproliferative activities. Dalton Trans 50:3990–4007. https://doi.org/10.1039/d0dt03962d
Patra A, Puschmann H, Manna SC (2021) Bidentate Schiff base coordinated square planer nickel(II) complexes: synthesis, crystal structure DFT/TD-DFT calculation and DNA/protein binding. Polyhedron 201:115146. https://doi.org/10.1016/j.poly.2021.115146
Kargar H, Ardakani AA, Tahir MN et al (2021) Synthesis, spectral characterization crystal structure and antibacterial activity of nickel(II) copper(II) and zinc(II) complexes containing ONNO donor Schiff base ligands. J Mol Struct 1233:130112. https://doi.org/10.1016/j.molstruc.2021.130112
Mahmoudi H, Bagherzadeh M, Ataie S et al (2020) Synthesis and X-ray crystal Structure of a molybdenum (VI) Schiff base Complex design of a new catalytic system for sustainable olefin epoxidation. Inorg Chim Acta 511:119775. https://doi.org/10.1016/j.ica.2020.119775
Ghosh AK, Purohit CS, Ghosh R (2018) Synthesis and structural characterization of a cobalt(III) complex with an (N, S, O) donor Schiff base ligand catechol oxidase and phenoxazinone synthase activities. Polyhedron 155:194–201. https://doi.org/10.1016/j.poly.2018.08.021
Maji AK, Chatterjee A, Khan S, Ghosh BK, Ghosh R (2017) Synthesis crystal structure catecholase and phenoxazinone synthase activities of a mononuclear cobalt(III) complex containing in situ formed tridentate N-donor Schiff base. J Mol Struct 1146:821–827. https://doi.org/10.1016/j.molstruc.2017.06.077
Gerdemann C, Eicken C, Krebs B (2002) The Crystal structure of catechol oxidase: new insight into the function of type-3 copper proteins. Acc Chem Res 35:183–191. https://doi.org/10.1021/ar990019a
Koval IA, Gamez P, Belle C, Selmeczi K, Reedijk J (2006) Synthetic models of the active site of catechol oxidase: mechanistic studies. Chem Soc Rev 35:814–840. https://doi.org/10.1039/b516250p
Adhikary J, Chakraborty P, Das S, Chattopadhyay T, Bauzà A, Chattopadhyay SK, Ghosh B, Mautner FA, Frontera A, Das D (2013) A combined experimental and theoretical investigation on the role of halide ligands on the catecholase-like activity of mononuclear nickel(ii) complexes with a phenol-based tridentate ligand. Inorg Chem 52:13442. https://doi.org/10.1021/ic401819t
Saswati AP, Majumder S et al (2018) Synthesis, structure, solution behavior, reactivity and biological evaluation of oxidovanadium(iv/v) thiosemicarbazone complexes. Dalton Trans 47:11358–11374. https://doi.org/10.1039/C8DT01668B
Yousef TA, El-Gammal OA, Ahmed SF, El-Reash GM (2014) Structural DFT and biological studies on Co(ii) complexes of semi and thiosemicarbazide ligands derived from diketo hydrazide. J Mol Struct 1076:227–237. https://doi.org/10.1016/j.molstruc.2014.07.053
Kasuga NC, Sekino K, Koumo C et al (2001) Synthesis structural characterization and antimicrobial activities of 4- and 6-coordinate nickel(ii) complexes with three thiosemicarbazones and semicarbazone ligands. J Inorg Biochem 84:55–65. https://doi.org/10.1016/S0162-0134(00)00221-X
Kumar SC, Ghosh AK, Chen J-D, Ghosh R (2017) Structurally characterized mononuclear Mn(II) complex Functional model for catecholase and phenoxazinone synthase activities. Inorg Chim Acta 464:49–54. https://doi.org/10.1016/j.ica.2017.04.043
de Oliveira JAF, da Silva MP, de Souza B et al (2016) Dopamine polymerization promoted by a catecholase biomimetic Cuii(μ-OH) Cuiicomplex containing a triazine-based ligand. Dalton Trans 45:15294. https://doi.org/10.1039/C6DT02032A
Paria S, Haldar P, Paine TK (2012) Oxidative carbon-carbon bond cleavage of a α-hydroxy ketone by a functional model of 2,4′-dihydroxyacetophenone dioxygenase. Angew Chem Int Ed 51:6195. https://doi.org/10.1002/anie.201201825
Biswas N, Saha S, Zangrando E et al (2021) Catecholase-like activity and theoretical study in solid state of a new Ru(III)-Schiff base complex. Acta Chim Slov 68:212–221. https://doi.org/10.17344/acsi.2020.6379
Saha S, Biswas N, Sasmal A et al (2018) Effect of temperature and ligand protonation on the electronic ground state in Cu(II) polymers having unusual secondary interactions: magnetic and catechol oxidase study. Dalton Trans 47:16102. https://doi.org/10.1039/C8DT02417K
Chirinos J, Ibarra D, Morillo A et al (2021) Synthesis, characterization and catecholase biomimetic activity of novel cobalt(II), copper(II), and iron(II) complexes bearing phenylene-bisbenzimidazole ligand. Polyhedron 203:115232–115238. https://doi.org/10.1016/j.poly.2021.115232
Titi A, Warad I, Tillard M et al (2020) Inermolecular interaction in [C6H10N3]2[CoCl4] complex: synthesis, XRD/HSA relation, spectral and catecholase catalytic analysis. J Mol Struct 1217:128422–128430. https://doi.org/10.1016/j.molstruc.2020.128422
Sarkar S, Lee H-In, (2020) Synthesis, structure, magnetic properties, and catecholase-like activity of a phenoxo bridged dinuclear cobalt(II) complex. Inorg Chim Acta 504:119437–119443. https://doi.org/10.1016/j.ica.2020.119437
Simandi LI, Simandi TL (1998) Kinetics and mechanism of the cobaloxime(II)-catalysed oxidative dehydrogenation of 3,5-di-tert-butylcatechol by O2. A functional oxidase model. J Chem Soc Dalton Trans. https://doi.org/10.1039/A803597K
Chakraborty P, Majumder S, Jana A, Mohanta S (2014) Syntheses, structures, catecholase activity, spectroscopy and electrochemistry of a series of manganese(III) complexes: role of auxiliary anionic ligand on catecholase activity. Inorg Chim Acta 410(65–75):65–75. https://doi.org/10.1016/j.ica.2013.10.013
Mitra M, Raghavaiah P, Ghosh R (2015) A mononuclear cobalt(III) complex and its catecholase activity. New J Chem 39:200. https://doi.org/10.1039/C4NJ01587H
Panja A, Shyamal M, Saha A, Mandal TK (2014) Methylene bridge regulated geometrical preferences of ligands in cobalt(III) coordination chemistry and phenoxazinone synthase mimicking activity. Dalton Trans 43:5433–5452. https://doi.org/10.1039/C3DT52597J
Kabsch W (2010).XDS ActaCrystD 66: 125–132.
Bruker (2008) SADABS SMART and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA
Burla MC, Caliandro R, Carrozzini B et al (2015) Crystal structure determination and refinement via SIR2014. J Appl Cryst 48:306–309. https://doi.org/10.1107/S1600576715001132
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8. https://doi.org/10.1107/S2053229614024218
Brandenburg K, Putz H (1999) DIAMOND crystal impact GbR. Bonn, Germany
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. Cryst Eng Comm 11:19–32. https://doi.org/10.1039/B818330A
Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, 2009. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, 2009.
GaussView, Version 5; Gaussian, Inc.: Wallingford, CT, 2009. GaussView, Version 5; Gaussian, Inc.: Wallingford, CT, 2009
Das LK, Biswas A, Kinyon JS et al (2013) Di-, tri-, and tetranuclear nickel(ii) complexes with oximato bridges: magnetism and catecholase-like activity of two tetranuclear complexes possessing rhombic topology. Inorg Chem 52:11744–11757. https://doi.org/10.1021/ic401020m
Nakamoto K (1997) Infrared and raman spectra of inorganic and coordination compounds, vol 23. Wiley, New York
Ray A, Banerjee S, Butcher RJ et al (2008) Two new end-on azido bridged dinuclear copper(II) and cobalt(III) complexes derived from the (E)-N′-((pyridin-2-yl)methylene) acetohydrazide Schiff base ligand: characterisation, crystal structures and magnetic study. Polyhedron 27:2409–2415. https://doi.org/10.1016/j.poly.2008.04.018
Banerjee S, Sen S, Basak S et al (2008) Two new pseudohalide-bridged Cu(II) complexes with a hydrazone ligand: syntheses, crystal structures and magnetic studies. Inorg Chim Acta 361:2707–2714. https://doi.org/10.1016/j.ica.2008.01.020
Biswas N, Khanra S, Sarkar A et al (2017) One new azido bridged dinuclear copper(II) thiosemicarbazide complex: synthesis, DNA/protein binding, molecular docking study and cytotoxicity activity. New J Chem 41:12996–13011. https://doi.org/10.1039/C7NJ01998J
Beyazit N, Catikkas B, Bayraktar S, Demetgul C (2016) Synthesis, characterization and catecholase-like activity of new Schiff base metal complexes derived from visnagin: theoretical and experimental study. J Mol Struct 1119:124–132. https://doi.org/10.1016/j.molstruc.2016.04.047
Dasgupta S, Majumder I, Chakraborty P et al (2017) Ligand flexibility controlled and solvent induced nuclearity conversion in cuII-based catecholase models: a deep insight by combined experimental and theoretical investigations. Eur J Inorg Chem. https://doi.org/10.1002/ejic.201600985
Alkam HH, Atiyah EM, Al-Shemary RK (2021) Nat Volatiles Essent Oils 8:12632
Chirinos J, Ibarra D, Morillo Á et al (2021) Synthesis, characterization and catecholase biomimetic activity of novel cobalt(II), copper(II), and iron(II) complexes bearing phenylene-bis-benzimidazole ligand. Polyhedron 203:115232. https://doi.org/10.1016/j.poly.2021.115232
Zhou J, Chen Z-F, Tan Y-S, Wang X-W, Tan Y-H, Liang H, Zhang Y (2004) Bis{1-[(E)-2-pyridinylmethylidene]semicarbazide}cobalt(II) diperchlorate monohydrate. Acta Cryst E 60:m519–m521. https://doi.org/10.1107/S1600536804007494
Chakraborty P, Majumder I, Banu KS et al (2016) Mn(II) complexes of different nuclearity: synthesis, characterization and catecholase-like activity. Dalton Trans 45:742–752. https://doi.org/10.1039/C5DT03659C
Majumder A, Goswami S, Batten SR et al (2006) Catalytic oxidation of 3,5-di-tert-butylcatechol by a manganese(III) 18-azametallacrown-6 compound: synthesis, crystal structure, fluorescence, magnetic and kinetic investigation. Inorg Chim Acta 359:2375–2382. https://doi.org/10.1016/j.ica.2006.01.045
Sarkar A, Chakraborty A, Adhikary A et al (2019) Exploration of catecholase-like activity of a series of magnetically coupled transition metal complexes of Mn, Co and Ni: new insights into the solution state behavior of Mn complexes. Dalton Trans 48:14164–14177. https://doi.org/10.1039/C9DT02399B
Reim J, Krebs B (1997) Synthesis, structure and catecholase activity study of dinuclear copper(II) complexes. J Chem Soc Dalton Trans. https://doi.org/10.1039/A704245K
Torelli S, Belle C, Gautier-Luneau I et al (2003) pH-Controlled change of the metal coordination in a dicopper(ii) complex of the ligand H-BPMP: crystal structures, magnetic properties, and catecholase activity. Inorg Chem 39:3526–3536. https://doi.org/10.1021/ic991450z
Neves A, Rossi LM, Bortoluzzi AJ (2002) Catecholase activity of a series of dicopper(II) complexes with variable Cu−OH(phenol) moieties. Inorg Chem 41:1788–1794. https://doi.org/10.1021/ic010708u
Mukherjee J, Mukherjee R (2002) Catecholase activity of dinuclear copper(II) complexes with variable endogenous and exogenous bridge. Inorg Chimica Acta 337:429–438. https://doi.org/10.1016/S0020-1693(02)01106-4
Dasgupta S, Adhikary J, Giri S et al (2017) Unveiling the effects of the in situ generated arene anion radical and imine radical on catecholase like activity: a DFT supported experimental investigation. Dalton Trans 46:5888–5900. https://doi.org/10.1039/C7DT00733G
Gentschev P, Möller N, Krebs B (2000) New functional models for catechol oxidases. Inorg Chim Acta 300–302:442–452. https://doi.org/10.1016/S0020-1693(99)00553-8
Seth P, Das LK, Drew MGB, Ghosh A (2012) Synthesis, crystal structure, and catecholase activity of three trinuclear heterometallic NiII2–MnIIComplexes derived from a Salen-type schiff base ligand. Eur J Inorg Chem. https://doi.org/10.1002/ejic.201101309
Mandal L, Mandal S, Mohanta S (2017) Syntheses, crystal structures, magnetochemistry and catechol oxidase activity of a tetracopper(ii) compound and a new type of dicopper(II)-based 1D coordination polymer. New J Chem 41:4689–4701. https://doi.org/10.1039/C7NJ00286F
Indira S, Vinoth G, Bharathi M et al (2019) Catechol oxidase and phenoxazinone synthase mimicking activities of mononuclear Fe(III) and Co(III) complexes of amino-bis(phenolate)-based mixed ligands: synthesis, spectral and electrochemical studies. Inorg Chim Acta 495:118988. https://doi.org/10.1016/j.ica.2019.118988
Majumder S, Mondal S, Lemonie P, Mohanta S (2013) Dinuclear mixed-valence CoIIICoII complexes derived from a macrocyclic ligand: unique example of a CoIIICoIIcomplex showing catecholase activity. Dalton Trans 42:4561–4569. https://doi.org/10.1039/C2DT32629A
Banerjee A, Guha A, Adhikary J et al (2013) Dinuclear cobalt(II) complexes of Schiff-base compartmental ligands: syntheses, crystal structure and bio-relevant catalytic activities. Polyhedron 60:102–109. https://doi.org/10.1016/j.poly.2013.05.014
Sanyal R, Kundu P, Rychagova E et al (2016) Catecholase activity of Mannich-based dinuclearCuIIcomplexes with theoretical modeling: new insight into the solvent role in the catalytic cycle. New J Chem 40:6623–6635. https://doi.org/10.1039/C6NJ00105J
Chakraborty P, Majumder I, Kara H et al (2015) Azido bridge mediated catecholase activity, electrochemistry and magnetic behavior of a dinuclear copper(II) complex of a phenol based ‘‘end-off” compartmental ligand. Inorg Chim Acta 436:139–145. https://doi.org/10.1016/j.ica.2015.07.038
Sahid M, Mantasha I, Khan S et al (2021) Elucidating the contribution of solvent on the catecholase activity in a mononuclear Cu(II) system: an experimental and theoretical approach. J Mol Struct 1244:130878. https://doi.org/10.1016/j.molstruc.2021.130878
Ferre FT, Resende JALC, Schultz J et al (2017) Catalytic promiscuity of mononuclear copper(II) complexes in mild conditions: catechol and cyclohexane oxidations. Polyhedron 123:293–304. https://doi.org/10.1016/j.poly.2016.11.045
DurigonDC PMM, Bortoluzzi AJ et al (2020) Cu(ii) complexes with tridentate sulfur and selenium ligands: catecholase and hydrolysis activity. New J Chem 44:15698–15707. https://doi.org/10.1039/D0NJ02806A
Biswas A, Das LK, Drew MGB et al (2012) Synthesis, crystal structures, magnetic properties and catecholase activity of double phenoxido-bridged penta-coordinated dinuclear nickel(ii) complexes derived from reduced schiff-base ligands: mechanistic inference of catecholase activity. Inorg Chem 51:7993–8001. https://doi.org/10.1021/ic202748m
Mandal B, Majee MC, Mandal D, Ganguly R (2020) Synthesis, structure, Hirshfeld surface analysis and catecholase activity of Ni(II) complex with sterically constrained phenol based ligand. J Mol Struct 1202:127340. https://doi.org/10.1016/j.molstruc.2019.127340
Das M, Nasani R, Saha M et al (2015) Nickel(II) complexes with flexible piperazinyl moiety: studies on DNA and protein binding and catecholase like properties. Dalton Trans 44:2299–2310. https://doi.org/10.1039/C4DT02675F
Vijayan P, Viswanathamurthi P, Velmurugan K et al (2015) Nickel(ii) and copper(ii) complexes constructed with N2S2 hybrid benzamidine–thiosemicarbazone ligand: synthesis, X-ray crystal structure, DFT, kinetico-catalytic and in vitrobiological applications. RSC Adv 5:103321–103342. https://doi.org/10.1039/C5RA18568H
Banu KS, Chattopadhyay T, Banerjee A et al (2009) Mono- and dinuclear manganese(III) complexes showing efficient catechol oxidase activity: syntheses, characterization and spectroscopic studies. Dalton Trans. https://doi.org/10.1039/B902498K
Mandal B, Haldar A, Saha R, Mandal D (2020) Mononuclear Mn(III) complex with sterically constrained phenol-based ligand: synthesis, structure and catecholase activity. J Mol Struct 1220:128723. https://doi.org/10.1016/j.molstruc.2020.128723
Adam MSS, Khalil A (2022) Nickel (II), copper (II), and vanadyl (II) complexes with tridentate nicotinoyl hydrazone derivative functionalized as effective catalysts for epoxidation processes and as biological reagents. J Taiwan Inst Chem Eng 132:104192. https://doi.org/10.1016/j.jtice.2021.104192
Adam MSS, Elsawy H, Sedky A, Makhlou MM (2022) Comparable catalytic and biological behavior of alternative polar dioxo-molybdenum (VI) Schiff base hydrazone chelates. J Taiwan Inst Chem Eng 136:104425. https://doi.org/10.1016/j.jtice.2022.104425
Anthony SMB, CW, Ron JP, et al (2016) Spectroscopic and TD-DFT studies on the turn-off fluorescent chemosensor based on anthraldehyde N(4) cyclohexyl thiosemicarbazone for the selective recognition of fluoride and copper ions. Polyhedron 109:7–18. https://doi.org/10.1016/j.poly.2016.01.021
Haribabi J, Jayalakshmi J, Arun A et al (2015) Synthesis, DNA/protein binding, molecular docking, DNA cleavage and in vitro anticancer activity of nickel(ii) bis(thiosemicarbazone) complexes. RSC Adv 5:46031–46049. https://doi.org/10.1039/C5RA04498G
McDonald AR, Gou Y, Van Vu V et al (2012) A mononuclear carboxylate-rich oxoiron(IV) complex a structural and functional mimic of TauD intermediate. J Chem Sci 3:1680. https://doi.org/10.1039/C2SC01044E
Manna S, Mukherjee J, Lloret F, Mukherjee R (2012) Modeling tyrosinase and catecholase activity using new m-Xylyl-based ligands with bidentate alkylamine terminal. Coordin Inorg Chem 51:13148. https://doi.org/10.1021/ic3013848
Biswas BK, Saha S, Biswas N et al (2020) Two copper (II) complexes derived from anthranilic acid and 4-iodoanthranilic acid Schiff bases: Structural elucidation, halogen bonding interactions and catalytic study using 3,5-DTBC. J Mol Struct 1217:128398. https://doi.org/10.1016/j.molstruc.2020.128398
Simandi TL, Simandi LI (1998) Solvent effect in the kinetics of cobaloxime(II)-catalyzed oxidative dehydrogenation of 3,5-di-tert- butylcatechol by O2. Reac Kinet Catal L 65:301–309. https://doi.org/10.1007/BF02475268
Dey SK, Mukherjee A (2014) The synthesis, characterization and catecholase activity of dinuclear cobalt(ii/iii) complexes of an O-donor rich Schiff base ligand. New J Chem 38:4985–4995. https://doi.org/10.1039/C4NJ00715H
Ghosh AK, Mitra M, Fathima et al (2016) Antibact.erial and catecholase activities of Co(III) and Ni(II) Schiff base complexes. Polyhedron 107:1–8. https://doi.org/10.1016/j.poly.2016.01.015
Mondal S, Pakhira B, Blake AJ et al (2016) Co(III) and Ni(II) complexes of an anthracene appended aroyl hydrazone: synthesis, crystal structures DNA binding and catecholase activity. Polyhedron 117:327–337. https://doi.org/10.1016/j.poly.2016.05.052
Roy S, Sarkar SK, Saha R (2018) Cobalt(ii), nickel(ii) and copper(ii) complexes of N-{(2-pyridyl)methyliden}-6-coumarin: characterization, DNA interaction, catecholase activity and theoretical interpretation. Inorg Chim Acta 482:659–668. https://doi.org/10.1016/j.ica.2018.05.001
Acknowledgements
M. Chowdhury acknowledges UGC, New Delhi, Government of India, for awarding Senior Research Fellowship (sr. no. 2121410140, ref. no. 21/12/2014 (II) EU-V). N. Biswas acknowledges CSIR, New Delhi, Government of India, for awarding Junior Research Fellowship (project no. 01/2537/11-EMR-II). C. Roy Choudhury acknowledges DSTFIST (project no. SR/FST/CSI-246/2012), New Delhi, Government of India, for instrumental support under capital heads.
Author information
Authors and Affiliations
Contributions
MC and NB did the whole work. SS wrote the main manuscript. EZ and CR performed the structural analysis. NS did the theoretical calculations. CRC wrote and checked the full manuscript. All authors reviewed the manuscript
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix A: Supplementary data
Appendix A: Supplementary data
CCDC 2,204,530 and 2,204,531 contain the supplementary crystallographic data for complexes 1 and 2, respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+ 44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk. Supplementary data associated with this article can be found, in the online version, at http://
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chowdhury, M., Biswas, N., Saha, S. et al. Structural investigation, theoretical DFT, Hirshfeld surface analysis and catalytic behaviour towards 3,5-DTBC oxidation of two cobalt(ii) complexes with semicarbazone Schiff base ligands. Transit Met Chem 48, 63–78 (2023). https://doi.org/10.1007/s11243-023-00523-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-023-00523-0