Abstract
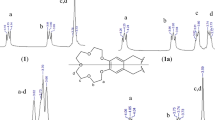
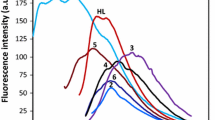
New double-armed benzo-15-crown-5 compound (L) was successfully synthesized from 4’,5’-bis(bromethyl)benzo-15-crown-5 with 2,2’-dipyridylamine. The synthesized host molecule (L), the dipyridylamine unit was able to coordinate Ni2+, Cu2+ and Ag+ metal cations, whereas the crown ether moiety bound with the alkali metal cations (Na+ and K+). The structures of the ligand (L), alkali metal complexes (NaL and KL2) and transition metal complexes ([NiLOAc], [CuLOAc] and [AgLNO3]) were characterized by spectroscopic methods. NMR and mass data provided exact evidence of complex formation through both coordination centers of the new ligand (L). Both parts (dipyridyl and crown ether) were linked to form a potential fluorescent-sensing compound (L) for metal cations. Therefore, to investigate the metal selectivity, different metal cations (Na+, Mg2+, K+, Ba2+, Cr3+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+ and Ag+) and the new sensing compound (L) fluorescence spectra were recorded. Coordinations with Zn2+, Fe3+ and Cu2+ induced obvious changes on their increasing concentrations in fluorescence spectra. Crown ethers, as representatives of supramolecular compounds, are also promising antibacterial active compounds because of their ionophoric features. Synthesized ligand (L) and complexes (NaL, KL2, [NiLOAc], [CuLOAc] and [AgLNO3]) also proved to be adjuvants that helped to overcome antimicrobial resistance in a range of bacteria and yeast. The antimicrobial activity of compounds was screened in vitro against some pathogenic Gram-positive bacteria, some Gram-negative bacteria and yeast.
Graphic Abstract











Similar content being viewed by others
References
Pedersen CJ (1967) J Am Chem Soc 89:7017–7036
Gokel GW, Leevy WM, Weber ME (2004) Chem Rev 104:2723–2750
Li J, Yim D, Jang WD, Yoon J (2017) Chem Soc Rev 46:2437–2458
Roelens S, Vacca A, Venturi C (2009) Chem Eur J 15:2635–2644
Antonioli B, Bray DJ, Clegg JK, Gloe K, Gloe K, Kataeva O, Lindoy LF, McMutrie JC, Steel PJ, Sumby CJ, Wenzel M (2006) Dalton Trans 40:4783–4794
Wu F, Tong H, Wang K, Wang Z, Li Z, Zhu X, Wong WY, Wong WK (2016) J Photochem Photobiol A 318:97–103
Youngme S, Chaichit N, Pakawatchai C, Booncoon S (2002) Polyhedron 21:1279–1288
Youngme S, Chaichit N, Koonsaeng N (2002) Inorg Chim Acta 335:36–42
Rauterkus MJ, Fakih S, Mock C, Puscasu I, Krebs B (2003) Inorg Chim Acta 350:355–365
Kralj M (2008) Tušek-Božić, Frkanec L. ChemMedChem 3:1478–1492
Calverly MJ, Dale J (1982) Acta Chem Scand 36B:241–247
Winkler B, Mau AWH, Dai L (2000) Phys Chem Chem Phys 2:291–295
Nithya C, Gnanalakshmi B, Pandian SK (2011) Mar Environ Res 71:283–294
Çiçek İ, Tunç T, Ogutcu H, Abdurrahmanoglu S, Günel A, Demirel N (2020) Chemistry Select 5:4650–4654
Koçoğlu S, Ogutcu H, Hayvalı Z (2019) Res Chem Intermed 45:2403–2427
Karakılıç E, Baran Ş, Öğütçü H, Akdemir A, Baran A (2020) rac- and meso-Cyclohexanoids: Their α-, β-glycosidases, antibacterial, antifungal activities and molecular docking studies. Arch Pharm. https://doi.org/10.1002/ardp.201900267
Liu Y, Han JR, Zhang HY (2004) Supramol Chem 16:247–254
Hayvali Z, Gündüz N, Kilic Z, Weber E (2000) Z Naturforsch 55b:975–981
Burlov AS, Tsukanov AV, Borodkin GS, Revinskii YV, Dubonosov AD, Bren VA, Garnovskii AD, Tsivadze AY, Minkin VI (2006) Russ J Gen Chem 76:992–996
Hayvalı Z, Köksal P (2013) J Incl Phenom Macrocycl Chem 76:369–378
Biernat JF, Cygan A, Luboch E, Simonov YA, Malinovski TI (1993) Bel’skii VK, Bolotina NF. J Inclus Phenom Mol 15:369–383
Şahin Gül D, Ogutcu H, Hayvalı Z (2020) J Mol Struct 1204:127569
Gao Y, Zhong RL, Xu HL, Sun SL, Su ZM (2015) RSC Adv 5:30107–30119
Keller BO, Sui J, Young AB, Whittal RM (2008) Anal Chim Acta 627:71–81
Tong H, Bell D, Tabei K, Siegel MM (1999) J Am Soc Mass Spectrom 10:1174–1187
Ghildiyal N (2017) nee Pant GJ, Rawat MSM, Singh K. Spectrochim Acta A 171:507–514
Alcock NW, Tracy VM, Waddington TC (1976) J Chem Soc Dalton Trans 21:2243–2246
Mathey Y, Greig DR, Shriver DF (1982) Inorg Chem 21:3409–3413
Wang AQ, Golden TD (2013) Int J Electrochem 2013:1–10
Zhongy DC, Chen ZF, Liu YC, Luo XJ, Barta C, Liang H (2010) J Coord Chem 63:3146–3154
Sánchez-Méndez A, Benito JM, de Jesús E, de la Mata FJ, Flores JC, Gómez R, Gómez-Sal P (2006) Dalton Trans 45:5379–5389
Ramadan S, Hambley TW, Kennedy BJ, Lay PA (2004) Inorg Chem 43:2943–2946
Koval IA, van der Schilden K, Schuitema AM, Gamez P, Belle C, Pierre JL, Luken M, Krebs B, Roubeau O (2005) Reedijk. J Inorg Chem 44:4372–4382
Şahin D, Yılmaz H, Hayvalı Z (2016) Res Chem Intermed 42:6337–6350
Sarı N, Şahin SÇ, Öğütcü H, Dede Y, Yalçın S, Altundas A, Doğanay K (2013) Spectrochim Acta A 106:60–67
Ramaswamy V, Cresence VM, Rejitha JS, Lekshmi MU, Dharsana KS, Prasad SP, Vijila HM (2007) Microb Infect 40:4–13
Ceker S, Ogutcu H, Meral S, Agar AA, Agar G (2019) Pak J Pharm Sci 32:2679–2686
Altundas A, Sarı N, Colak N, Ögütcü H (2010) Med Chem Res 19:576–588
Nartop D, Hasanoğlu Özkan E, Gündem M, Çeker S, Ağar G, Öğütcü H, Sarı N (2019) J Mol Struct 1195:877–882
Nartop D, Demirel B, Güleç M, Hasanoğlu Özkan E, Kurnaz Yetim N, Sarı N, Çeker S, Öğütcü H, Ağar G (2020) Novel polymeric microspheres: Synthesis, enzyme immobilization, antimutagenic activity and antimicrobial evaluation against pathogenic microorganisms. J Biochem Mol Toxic. https://doi.org/10.1002/jbt.22432
Nartop D, Sarı N, Öğütcü H (2014) Chin J Inorg Chem 30:921–929
Çınarlı M, Yüksektepe Ataol Ç, Bati H, Güntepe F, Ögütçü H, Büyükgüngör O (2019) Inorg Chim Acta 484:87–94
Mishra L, Singh VK (1993) Indian J Chem 32A:446–449
Reichling J, Koch C, Stahl-Biskup E, Sojka C, Schnitzler P (2005) Rev Bras Ciên Vet 18:62–66
Acknowledgements
The authors gratefully acknowledge the financial assistance of the Scientific and Technical Research Council of Turkey (TUBITAK), grant No: TBAG 210T122 and Ankara University grant No: 17B0430004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koçoğlu, S., Hayvalı, Z. & Ogutcu, H. A polydentate ligand based on 2,2’-dipyridylamine unit linked benzo-15-crown-5; alkali and transition metal complexes; photoresponsive ligand; antimicrobial evaluation against pathogenic microorganisms. Transit Met Chem 46, 509–522 (2021). https://doi.org/10.1007/s11243-021-00469-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-021-00469-1