Abstract
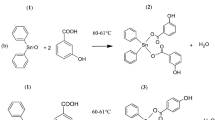
New double-armed crown ether ligands linked to pyridine derivatives have been synthesized and characterized. These macrocyclic ligands (1–5) have been synthesized by the reactions of 4′,5′-bis(bromomethyl)benzo-15-crown-5 with 3-hydroxy pyridine derivatives. A series of Na+, K+ and Ag+ complexes (1a–5a, 1b–5b and 1c–5c) of the macrocyclic ligands have been prepared from sodium perchlorate, sodium picrate, potassium iodide, potassium picrate and silver nitrate salts, respectively. The most suitable cation Na+ is bound to the 15-crown-5 cavity and 1:1 “filling complexes” are formed (1a–5a) while the K+ cation interacts with the crown ether cavity and forms sandwich-type complexes (1b–5b). The Ag+ complexes (1c–5c) have been obtained with a pyridine moiety of the new crown ethers. New ligands undergo photophysical changes when bonding the cation. The influence of metal cations such as Na+, Li+, K+, Fe3+, Cu2+, Ca2+, Ba2+ and Al3+ on the spectroscopic properties of the pyridine linked to the double-armed crown ether moiety was investigated in EtOH solution by means of absorption and emission spectrometry. The prepared compounds (1–5, 1a–5a, 1b–5b and 1c–5c) were evaluated for antibacterial and antifungal activities against pathogenic microorganisms. The results show that the antimicrobial activity of the synthesized compounds varying a degree of inhibitory effects on the growth of different tested pathogenic strains.












Similar content being viewed by others
References
C.J. Pedersen, J. Am. Chem. Soc. 89, 7017 (1967)
N.S. Poonia, A.V. Bajaj, Chem. Rev. 79, 389 (1979)
F. Vögtle, E. Weber, in Crown Ethers and Analogs, ed. by S. Patai, Z. Rappoport (Wiley, Chichester, 1989), p. 207
Z. Hayvali, N. Gündüz, Z. Kiliç, E. Weber, J. Prakt. Chem. 341, 568 (1999)
C. Sousa, C. Freire, B. De Castro, Molecules 8, 894 (2003)
D. Liu, K. Tang, W. Liu, C. Su, X. Yan, M. Tan, Y. Tang, Dalton Trans. 39, 9763 (2010)
K. Sako, T. Kakehi, S. Nakano, H. Oku, X.F. Shen, T. Iwanaga, M. Yoshikawa, K. Sugahara, S. Toyota, H. Takemura, T. Shinmyozu, M. Shiotsuka, H. Tatemitsu, Tetrahedron Lett. 55, 749 (2014)
B. Valeur, I. Leray, Coord. Chem. Rev. 205, 3 (2000)
Q.Z. Yang, L.Z. Wu, H. Zhang, B. Chen, Z.X. Wu, L.P. Zhang, C.H. Tung, Inorg. Chem. 43, 5195 (2004)
E.N. Ushakov, M.V. Alfimov, S.P. Gromov, Macroheterocycles 3, 189 (2010)
H.S. Seo, S.H. Lee, J. Fluoresc. 21, 747 (2011)
L. Zhao, X. Chen, F. Guo, B. Gou, C. Yang, W. Xia, J. Lumin. 145, 486 (2014)
J.-P. Malval, R. Lapouyade, Helv. Chim. Acta 84, 2439 (2001)
S.K. Kim, M.Y. Bang, S.-H. Lee, K. Nakamura, S.-W. Cho, J. Yoon, J. Incl. Phenom. Macrocycl. Chem. 43, 71 (2002)
D. Şahin, H. Yılmaz, Z. Hayvalı, Res. Chem. Intermed. 42, 6337 (2016)
D. Şahin, Y. Süzen, Z. Hayvalı, Hetoroatom Chem. 25, 43 (2014)
H. Güler, Z. Hayvali, H. Dal, T. Hökelek, Polyhedron 31, 688 (2012)
K.K. Haldar, T. Sen, A. Patra, J. Phys. Chem. C 114, 4869 (2010)
Z. Hayvalı, H. Güler, H. Öğütcü, N. Sarı, Med. Chem. Res. 23, 3652 (2014)
G.W. Gokel, W.M. Leevy, E. Weber, Chem. Rev. 104, 2723 (2004)
M. Kralj, L. Tusek-Bozic, L. Frkanec, Chem. Med. Chem. 3, 1478 (2008)
P.L. Caradoc-Davies, L.R. Hanton, W. Henderson, J. Chem. Soc. Dalton Trans. 19, 2749 (2001)
Y. Kang, S.S. Lee, K.-M. Park, S.H. Lee, S.O. Kang, J. Ko, Inorg. Chem. 40, 7027 (2001)
C. Seward, J. Chan, D. Song, S. Wang, Inorg. Chem. 42, 1112 (2003)
W. Sun, Y. Cui, H. Liu, H. Zhao, W. Zhang, J. Mol. Struct. 1026, 133 (2012)
T. Nakamura, K. Takeuchi, JP Patent 2003-238832A, 2003
N. Kinarivala, P.C. Trippier, Tetrahedron Lett. 55, 5386 (2014)
T.F. Spande, H.M. Garraffo, M.W. Edwards, H.J.C. Yeh, L. Pannell, J.W. Daly, J. Am. Chem. Soc. 114, 3475 (1992)
J.K. Lynch, M.W. Holladay, K.B. Ryther, H. Bai, C.N. Hsiao, H.E. Morton, D.A. Dickman, W. Arnold, S.A. King, Tetrahedron-Asymmetr. 9, 2791 (1998)
J. Li, D. Yim, W.-D. Jang, J. Yoon, Chem. Soc. Rev. 46, 2437 (2017)
V.K. Gupta, S. Chandra, S. Agarwal, Indian J. Chem. 42, 813 (2003)
M.J. Calverley, J. Dale, Acta Chem. Scand. B. 36, 241 (1982)
B. Winkler, A.W.-H. Mau, L. Dai, Phys. Chem. Chem. Phys. 2, 291 (2000)
A. Bilgin, B. Ertem, P. Dinc Agın, Y. Gok, S. Karslıoglu, Polyhedron 25, 3165 (2006)
H. Öğütcü, N.K. Yetim, E.H. Özkan, O. Eren, G. Kaya, N. Sarı, A. Dişli, Pol. J. Chem. Technol. 19, 74 (2017)
C. Nithya, B. Gnanalakshmi, S.K. Pandian, Mar. Environ. Res. 71, 283 (2011)
U. Schillinger, F.K. Lucke, Appl. Environ. Microbiol. 55(8), 1901 (1989)
M. Balouiri, M. Sadiki, K.S. Ibnsouda, J. Pharm. Anal. 6, 79 (2016)
S. Magaldi, S. Mata-Essayag, C. Hartung de Capriles, C. Perez, M.T. Colella, C. Olaizola, Y. Ontiveros, Int. J. Infect. Dis. 8, 39 (2004)
C. Valgas, S.M. De Souza, E.F.A. Smânia, A. Smânia Jr., Braz. J. Microbiol. 38, 369 (2007)
Y. Xiang, X. Liu, C. Mao, X. Liu, Z. Cui, X. Yang, K.W.K. Yeung, Y. Zheng, S. Wu, Mater. Sci. Eng. C 85, 214 (2018)
Z. Liu, Y. Zhu, X. Liu, K.W.K. Yeung, S. Wu, Colloids Surf. B 151, 165 (2017)
Y. Zhu, X. Liu, K.W.K. Yeung, P.K. Chu, S. Wu, Appl. Surf. Sci. 400, 14 (2017)
C. Mao, Y. Xiang, X. Liu, Z. Cui, X. Yang, K.W.K. Yeung, H. Pan, X. Wang, P.K. Chu, S. Wu, ACS Nano 11, 9010 (2017)
E. Bozkır, N. Sarı, H. Ögütcü, J. Inorg. Organomet. Polym. Mater. 22, 1146 (2012)
N. Sarı, N. Pişkin, H. Öğütcü, N. Kurnaz, Med. Chem. Res. 22, 580 (2013)
D. Nartop, N. Sarı, H. Öğütcü, Chin. J. Inorg. Chem. 30, 921 (2014)
A. Altundas, N. Sarı, N. Colak, H. Ögütcü, Med. Chem. Res. 19, 576 (2010)
D. Nartop, N. Sarı, A. Altundas, H. Ögütcü, J. Appl. Polym. Sci. 125, 1796 (2012)
M. Barboiu, A. Mefrfre, Y.-M. Legrand, E. Petit, L. Marin, M. Pinteala, A.V.D. Lee, Supramol. Chem. 26, 223 (2014)
N.S. Poonia, P. Bagdi, K.S. Sidhu, J. Incl. Phenom. 4, 43 (1986)
B. Antonioli, D.J. Bray, J.K. Clegg, K. Gloe, K. Gloe, O. Kataeva, L.F. Lindoy, J.C. McMurtrie, P.J. Steel, C.J. Sumby, M. Wenzel, Dalton Trans. 40, 4783 (2006)
G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd edn. (Wiley, Chichester, 2001)
D. Lin-Vien, N.B. Colthup, W.G. Fateley, J.G. Graselli, The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules (Academic Press, San Diego, 1991)
N. Ghildiyal, G.J. Nee Pant, M.S.M. Rawat, K. Singh, Spectrochim. Acta A 171, 507 (2017)
Y. Liu, J.R. Han, H.Y. Zhang, Supramol. Chem. 16, 247 (2004)
Z. Hayvalı, P. Köksal, J. Incl. Phenom. Macrocycl. Chem. 76, 369 (2013)
C.J. Pedersen, H.K. Frensdorff, Angew. Chem. Internat. Edit. 11, 16 (1972)
P.R. Mallison, M.R. Truter, J. Chem. Soc. Perkin 2, 1818 (1972)
V.W. Bhagwat, H. Manohar, N.S. Poonia, Inorg. Nucl. Chem. Lett. 17, 207 (1981)
R. Ziessel, L. Bonardi, P. Retailleau, G. Ulrich, J. Org. Chem. 71, 3093 (2006)
S. Imama-Reja, N. Kumar, R. Sachdeva, V. Bhalla, M. Kumar, RSC Adv. 3, 17770 (2013)
V. Bojinov, N. Georgiev, J. Chem. Technol. Metall. 46, 3 (2011)
F.R.F. Dias, J.S. Novais, T.A. do Nascimento Santos Devillart, W.A. da Silva, M.O. Ferreira, R.S. Loureiro, V.R. Campos, V.F. Ferreira, M.C.B.V. de Souza, H.C. Castro, A.C. Cunha, Eur. J. Med. Chem. 156, 1 (2018)
N.B. Reddy, G.V. Zyryanov, G.M. Reddy, A. Balakrishna, A. Padmaja, V. Padmavathi, C.S. Reddy, J.R. Garcia, G. Sravya, J. Heterocycl. Chem. https://doi.org/10.1002/jhet.3435
Z. Xu, X. Wang, X. Liu, Z. Cui, X. Yang, K.W.K. Yeung, J.C. Chung, P.K. Chu, S. Wu, ACS Appl. Mater. Interfaces 9, 39657 (2017)
X. Xie, C. Mao, X. Liu, Y. Zhang, Z. Cui, X. Yang, K.W.K. Yeung, H. Pan, P.K. Chu, S. Wu, ACS Appl. Mater. Interfaces 9, 26417 (2017)
I. Sondi, B. Salopek-Sondi, J. Colloid Interface Sci. 275, 177 (2004)
A. Altundas, Y. Erdogan, H. Ögütcü, H.E. Kizil, G. Agar, Fresenius Environ. Bull. 25, 5411 (2016)
Acknowledgements
The authors gratefully acknowledge the financial assistance of the Scientific and Technical Research Council of Turkey (TUBITAK), Grant No. TBAG 210T122, and Ankara University Grant No. 17B0430004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koçoğlu, S., Ogutcu, H. & Hayvalı, Z. Photophysical and antimicrobial properties of new double-armed benzo-15-crown-5 ligands and complexes. Res Chem Intermed 45, 2403–2427 (2019). https://doi.org/10.1007/s11164-019-03741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03741-3