Abstract
Six mixed metal complexes with 3-aminopyridine (3-ampy) as a co-ligand have been synthesized: catena-{[M(μ2-3-ampy)(H2O)4]SO4·H2O} (M=Ni (1) and Co (2)), [Co(3-ampy)4(NCS)2] (3), [Co(3-ampy)2(NCS)2] (4), [Co(3-ampy)4(N3)2] (5) and mer-[Co(3-ampy)3(N3)3] (6), (NCS−=isothiocyanate ion, N3− azide ion), and characterized by physio-chemical and spectroscopic methods as well as single crystal X-ray and powder diffraction. In the isostructural complexes 1 and 2 single μ2-3-ampy links the Ni(II) and Co(II) centers into polymeric chains. The mononuclear Co(II) and Co(III) pseudohalide complexes 3–6 reveal only terminal 3-ampy ligands. The 3-ampy ligands form supramolecular hydrogen bonded systems via their NH2-groups and non-covalent π-π ring-ring interactions via their pyridine moieties. Thermoanalytical properties were investigated for 1–3.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aminopyridine ligands have been extensively studied in the synthesis of many coordination metal compounds ranging from simple mononuclear to coordination polymeric compounds (CPs) with different dimensionality. The construction of CPs is attributed to the intriguing structural diversity and the dual functionality of these ligands, which may lead to the formation of 1D or 3D structures [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19], especially in the presence of other potentially bridging ligands such as pseudohalides [1,2,3, 13]. Another feature incorporated in this class of compounds is their affinity to show different hydrogen bonds, N–H⋯X between the amino NH and a coordinated pseudohalide (X), which tends to extend the structure from 1D to a 3D network. Extra stability can also be generated through π-π stacking interactions between the aromatic pyridyl ligands, which play an important role in stabilizing the resultant polymeric architectures [1,2,3, 9, 15, 16].
3-Aminopyridine (3-ampy) as one of the aminopyridine series is a simple molecule which can act as mondentate ligand by binding the metal ions in the most common binding mode via the most basic N-pyridyl nitrogen (II) [2, 7, 15, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] or the least likely binding site of the amino group (III) [33, 34]. The latter bonding mode can also be achieved through the N-amino donor site of the pyridinium ion (IV) [24, 38, 39]. Moreover, the molecule can also, simultaneously bridging two metal ions via the N-pyridyl and N-amino donor atoms (I) [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Different binding modes of the 3-aminopyridine ligand are summarized in Scheme 1. In some complexes, the 3-ampy was reported to behave as monoprotonated 3-aminopyridinium cation [24, 38, 40,41,42,43] or as doubly protonated 3-ammonium pyridinium dication [44, 46]. In an attempt to explore the coordination chemistry (nuclearity, CPs, dimensionality, hydrogen bonding and non-covalent interactions) of 3-ampy with metal ions (Co(II), Ni(II), and Co(III)) in the presence of potentially bridging pseudohalide ligands (N3−=azide anion and NCS−=thiocyanate anion), the following study was undertaken.
Experimental
Materials and physical measurements
3-aminopyridine was purchased from Aldrich. All other materials were reagent grade quality. Infrared spectra of the solid complexes were performed on a Bruker Alpha P (platinum-ATR-cap). UV-Vis-NIR spectra were recorded with a LS950 Perkin-Elmer Lambda-spectrometer. Thermal analyses were performed on solid samples with using NETSCH STA (N2 atmosphere; heating rate 10 °C/min). PXRD measurements of the microcrystalline bulk material were performed with a Bruker D8 Advance powder diffractometer. Elemental CHN microanalyses were carried out with an Elementar Vario EN3 analyzer.
Synthesis of the compounds
Synthesis of catena-{[Ni(μ2-3-ampy)(H2O)4]SO4·H2O} (1)
NiSO4.6H2O (0.263 g, 1 mmol), and 3-aminopyridine (0.095 g, 1 mmol) were dissolved in 80 ml H2O at 90 °C. Upon slow cooling to RT blue crystals of 1 were separated after 2 days (yield: 0.24 g, 71%). Anal. Calcd for C5H16N2NiO9S (338.95 g/mol): 17.7% C; 4.8% H; 8.3% N; Found: 17.6% C; 4.8% H; 8.2% N; IR (ATR, cm−1): 3162 (s, br), 1655 (w), 1612 (w), 1593 (w), 1560 (w), 1495 (w), 1455 (w), 1262 (w), 1077 (s), 1043 (s), 1021 (s), 905 (w), 836 (w), 810 (m), 778 (w), 745 (w), 697 (m), 650 (m), 609 (m), 559 (m), 423 (w).
Synthesis of catena-{[Co(μ2-3-ampy)(H2O)4]SO4·H2O} (2)
A procedure similar to that described for 1 was used to synthesize the pink needle-shaped crystals of 2 using CoSO4·7H2O (0.28 g, 1 mmol) instead of Ni(II) salt (yield: 0.26 g, 77%). Anal. Calcd for C5H16CoN2O9S (339.19 g/mol): 17.7% C; 4.8% H; 8.3% N; Found: 17.5% C; 4.9% H; 8.4% N; IR (ATR, cm−1): 3200 (s, br), 1655 (w), 1611 (w), 1591 (w), 1495 (w), 1454 (w), 1262 (w), 1077 (s), 1010 (s), 905 (w), 810 (w), 724 (w), 680 (m), 649 (m), 613 (s), 554 (m), 524 (m), 418 (w).
Synthesis of [Co (3-ampy)4 (NCS)2] (3)
A mixture of Co(NO3)2·6H2O (0.29 g, 1 mmol), KSCN (0.19 g, 2 mmol) and 3-aminopyridine (0.38 g, 4 mmol) were dissolved in 130 ml of distilled H2O at 75° C for about 15 min. The hot solution was filtered while hot and then slowly cooled to RT. After six days, fine orange-red needle-shaped crystals were obtained (yield: 0.35 g, 64%). Anal. Calcd for C22H24CoN10S2 (551.56 g/mol): 47.9% C; 4.4% H; 25.4% N; Found: 47.7% C; 4.3% H; 25.6% N; IR (ATR, cm−1): 3405 (w), 3329 (w), 3283 (w), 3191 (w), 3040 (w), 2083 (vs), 1621 (s), 1579 (s), 1485 (m), 1441 (s), 1342 (w), 1292 (m), 1257 (w), 1190 (w), 1128 (m), 1084 (w), 1047 (m), 1023 (w), 961 (w), 939(w), 895 (w), 846 (w), 816 (m), 795 (m), 737 (w), 696 (m), 640 (s), 547 (m), 516 (w), 479 (w), 415 (w).
Synthesis of [Co (3-ampy)2 (NCS)2] (4)
KSCN (0.39 g, 4 mmol) and 3-aminopyridine (0.19 g, 2 mmol) were added to a solution containing CoCl2.6H2O (0.48 g, 2 mmol) dissolved in distilled H2O (120 ml). The mixture was heated to a temperature of 80–90° C for 2 h and then filtrated while hot. The solution was stored at 40° C for one day where the dark blue crystals which separated were collected by filtration and air-dried (yield: 0.44 g, 60%). Anal. Calcd for C12H12CoN6S2 (363.33 g/mol): 39.7% C; 3.3% H; 23.1% N; Found: 39.4% C; 3.5% H; 23.1% N; IR (ATR, cm−1): 3446 (w), 3343 (w), 2090 (s), 2071 (vs), 1620 (m), 1578 (m), 1491 (m), 1444 (m), 1351 (m), 1311 (m), 1273 (m), 1189 (m), 1134 (m), 1058 (w), 893 (m), 811 (w), 757 (m), 712 (m), 693 (w), 658 (m), 609 (s), 547 (w), 421 (w).
Synthesis of [Co(3-ampy)4(N3)2] (5) and [Co(3-ampy)3 (N3)3] (6)
A mixture of Co(NO3)2·6H2O (0.29 g, 1 mmol), NaN3 (0.13 g, 2 mmol) and 3-aminopyridine (0.38 g, 4 mmol) were dissolved in distilled H2O (140 ml) at 95 °C, and the hot solution was filtered and allowed to stand at RT. The pink prism-shaped crystals which separated in the following day were collected by filtration and dried in air (yield: 0.25 g, 48%). Anal. Calcd for C20H24CoN14 (519.46 g/mol): 46.2% C; 4.7% H; 37.8% N; Found: 46.0% C; 4.6% H; 37.9% N; IR (ATR, cm−1): 3445 (w), 3376 (w), 3319 (w), 3216 (w), 2052 (vs), 1628 (w), 1774 (s), 1487 (s), 1439 (s), 1342 (w), 1298 (m), 1261 (w), 1192 (w), 1134 (w), 1091 (w), 1052 (w), 1021 (w), 978 (w), 914 (w), 880 (w), 842 (w), 802 (s), 704 (s), 639 (m), 549 (w), 525 (w), 414 (w).
Slow evaporation of the mother liquor of 5 at RT resulted in the separation of fine green needle-shaped crystals of [Co(3-ampy)3(N3)3] (6) after three weeks. During this period, Co(II) was oxidized to Co(III) upon by aerial oxidation (yield: 0.18 g, 39%). Anal. Calcd for C15H18CoN15 (467.37 g/mol): 38.5% C; 3.9% H; 45.0% N; Found: 38.3% C; 3.8% H; 45.2% N; IR (ATR, cm−1): 3422 (w), 3323 (w), 3213 (w), 2004 (vs), 1719 (w), 1603 (w), 1580 (m), 1488 (w), 1447 (w), 1356 (w), 1295 (w), 1264 (w), 1192 (w), 1128 (w), 1086 (w), 1059 (w), 1025 (w), 908 (w), 879 (w), 799 (m), 697 (m), 655 (w), 602 (w), 585 (w), 544 (w), 443 (w).
X-ray crystal structure analysis
The X-ray single-crystal data of the six title compounds were collected on a Bruker-AXS APEX II CCD diffractometer at 100(2) K. The crystallographic data, conditions retained for the intensity data collection and some features of the structure refinements are listed in Table 1. Data collections were performed with Mo-Kμ radiation (λ = 0.71073 Å); data processing, Lorentz-polarization and absorption corrections were performed using APEX and the SADABS computer programs [47, 48]. The structures were solved by direct methods and refined by full-matrix least-squares methods on F2, using the SHELX [49, 50] program library. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were located from difference Fourier maps, assigned with isotropic displacement factors. Geometrical constraints (HFIX) were applied only for H atoms bonded to C atoms. Further programs used: Mercury, PLATON and ToposPro [51,52,53]. Packing plots are given in the supplementary section (Figs. S1-S6).
Results and discussion
Synthesis, IR and UV-VIS spectroscopy
The synthesis of the complexes 3–5 was straightforward. Reactions of corresponding metal(II) salts with 3-ampy and KSCN (3 and 4) or NaN3 (5) in aqueous or aqueous methanol, afforded the corresponding title compounds. Complexes 1 and 2 were obtained by reaction of equimolar amounts of metal(II) sulfate hydrates with 3-ampy from aqueous solutions. The Co(III) azido complex 6 was obtained as by-product in the synthesis of 5 due to air oxidation of the Co(II) in the mother liquor of 5. The phase purity of the bulk material of solid compounds was checked by XRPD patterns of 1–6 (Figs S7-S12, supplementary section).
In addition to the vibrations of the pyridine moiety of the 3-ampy molecule in the complexes, the IR spectra show the characteristic bands of the pseudohalide ligands. The strong or very strong absorption bands of νas(SCN) and νas(N3) in complexes 3–6 are found at 2083 cm−1 (3), at 2090 and 2071 cm−1 (4), at 2052 cm−1 (5) and at 2004 cm−1 (6), respectively. The weak to medium strong vibrations of the –NH2 group occur in the region 3100–3500 cm−1. In case of 1 and 2, these bands are buried by the broad band at ~ 3180 cm−1 arising from O–H vibrations of the lattice water molecule and aqua ligands. In 1 and 2, the ionic nature of the sulfate group is indicated by the appearance of a very strong broad band (v3) centered around 1077 cm−1 (1), 1077 cm−1 (2) and a sharp band (ν4) at 609 cm−1 for 1 and 613 cm−1 for 2 [2, 28, 29, 54].
The UV–VIS spectrum of solid Ni(II) complex 1 reveals three broad bands centered at 1118 nm, 654 nm and 312 nm corresponding to the electronic transitions 3T2g ← 3A2g, 3T1g(F) ← 3A2g, 3T1g(P) ← 3A2g, respectively, for Ni(II) in octahedral geometry [55,56,57,58]. The UV-VIS spectra of the solid Co(II) complexes 2, 3 and 5 exhibit three absorption bands at 1210, 505 and 335 nm for 2, at 1075, 500 and 372 nm for 3, and at 1200, 520 and 372 nm for 5. These bands are assigned to the electronic transitions 4T2g(F) ← 4T2g(F), 4T2g(P) ← 4T1g(F), and 4A2g ← 4T1g(F), respectively, for Co(II) in octahedral environment [55,56,57,58]. The solid UV-VIS spectrum of Co(II) complex 4 shows absorption bands at 1340 nm, 574 nm and 340 nm, which corresponds to the 4T2g ← 4T1g, 4A2g ← 4T1g(F) and 4T1g(P) ← 4T1g(F), respectively, for Co(II) in tetrahedral environment. The UV–VIS spectrum of solid Co(III) complex 6 shows a broad absorption band centered at ~ 625 nm corresponding to 5E ← 5T2 transition in octahedral Co(III) in weak ligand field environment [55,56,57,58].
Description of the structures
catena-{[M(μ2-3-ampy)(H2O)4]SO4·H2O} M = Ni (1) and Co (2)
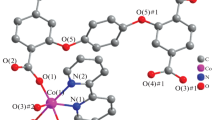
Perspective views of the isostructural complexes 1 and 2 are given in Figs. 1 and 2, respectively, with main bond parameters are listed in Table S1. The structures consist of [M(3-ampy)(H2O)4]2+ cations, sulfate anions, and lattice water molecules packing. The complex cations contain two crystallographically different metal centers. M(1) atom is trans coordinated by two hetero N atoms of 3-ampy ligands [M1-N1 = 2.0979(17) for 1 and 2.1446(14) Å for 2], whereas M(2) is trans coordinated by two amino N atoms of two 3-ampy molecules [M2-N2 = 2.1729(18) for 1 and 2.2253(15) Å for 2], in addition to four aqua molecules for each metal center [M–O from 2.0428(15) to 2.0819(15) Å for 1 and from 2.0546(13) to 2.1100(13) Å for 2]. The 3-aminopyridine behaves as a N,N’-bidentate bridging ligand generating 1D polymeric chains. The metal centers are located at inversion centers. The intrachain M⋯M distance is 6.7326(4) for 1 and 6.6949(5) Å for 2 and the M2-N2-C4 angles are 116.96(13) for 1 and 116.27(11)° for 2. The interchain metal–metal separations are 6.7706(5), 7.5852(6), 7.8462(6) Å for 1 and 6.7734(4), 7.6067(4), 7.8728(4) for Å 2. There are different types of hydrogen bonds consolidating the crystal structure by generating a supramolecular 3D network (Figs. S1 and S2): (1) O–H⋯O bonds between oxygen atoms of aqua molecules and lattice water; (2) O–H⋯O hydrogen bonds between oxygen atoms of the SO42− anion and oxygen atoms of aqua molecules and/or lattice water molecules (Table S3). Compounds 1 and 2 are isostructural to Cd(II) [2] and Cu(II) [12] analogous compounds.
[Co(3-ampy)4(NCS)2] (3) and [Co(3-ampy)2(NCS)2] (4)
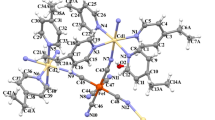
Coordination figures with partial atom labeling schemes for 3 and 4 are given in Figs. 3 and 4, the corresponding packing views are shown in Figs. S3 and S4, respectively, and main bond parameters are summarized in Table S2. In the monomeric compound 3, the Co(II) center has a trans-octahedral geometry formed by four terminal 3-ampy ligands and two terminal N-coordinated isothiocyanate anions, whereas the distorted tetrahedron around Co(II) in complex 4 is formed by two 3-ampy ligands and two terminal N-coordinated isothiocyanate anions. The Co–N bond lengths in 3 vary from 2.0831(14) to 2.2119(14) Å, and in 4 from 1.941(4) to 2.016(3) Å. The Co–N-C and N–C–S bond angles vary from 156.89(13) to 170.7(4)° and from 177.28(15) to 179.5(5)°; the N–C bonds vary from 1.155(7) to 1.160(7) and C–S bonds from 1.626(6) to 1.6386(16) Å.
[Co(3-ampy)4(N3)2] (5) and [Co(3-ampy)3(N3)3] (6)
Coordination figures with partial atom labeling schemes for 5 and 6 are given in Figs. 5 and 6, packing views are represented in Figs. S5 and S6, respectively, and main bond parameters are summarized in Table S3. In the monomeric compound 5, the Co(II) center has a trans-octahedral geometry formed by four terminal 3-ampy ligands and two terminal azide anions, whereas the mer-octahedral configuration of Co(III) in complex 6 is formed by three 3-ampy ligands and three terminal azide anions. The Co–N bond lengths in 5 vary from 2.107(3) to 2.238(3) Å, and in 6 from 1.945(2) to 1.978(2) Å. The Co–N–N and N–N–N bond angles vary from 122.1(3) to 130.0(3)° and from 175.8(3) to 178.3(4)°; the Nα-Nβ from 1.182(5) to 1.205(3) Å and Nβ-Nγ from 1.155(3) to 1.182(5) Å (with azido Nα atom bonded to metal center). The amino group forms hydrogen bonds of type N–HN to non-coordinated N4 and N8 atoms of the azide anions (Table S4). Non-covalent π⋯π ring⋯ring interactions are observed between neighbouring pyridine rings (Table S5).
Analysis of hydrogen bonded networks with ToposPro 5.4.0.2 program reveal 2M4-1 for 1 and 2, sql for 3, pcu for 5 and hex topology for 6, respectively.
Thermoanalytical behaviour of compounds 1–3
The thermogravimetric heating curves for compounds 1 and 2 are shown in Fig. 7. The Ni(II) compound 1 shows the following steps with weight losses: step 1: Δm = 26.63% (80–245° C); step 2: Δm = 6.60% (245–405 °C); step 3: Δm = 31.94% (405–550 °C); step 4: Δm = 6.96% (550–975 °C). The Co(II) compound 2 shows the following steps with weight losses: step 1: Δm = 26.38% (70–195 °C); step 2: Δm = 8.58% (195–340 °C); step 3: Δm = 13.43% (340–442 °C); step 4: Δm = 26.45% (442–975 °C). The first step of weight loss is accompanied with a sharp DSC signal at 174.2C and 138.8 °C for 1 and 2, respectively. The weight losses of 26.63% and 26.38% matches quite well with release of one lattice water molecule and four aqua ligands (theoretical: 26.58% and 26.56%) for 1 and 2, respectively. The anhydrous products show further steps of weight losses by releasing the 3-ampy ligand and the decomposition of the sulfate anion. The residual mass of the Ni(II) sample at 975 °C is 27.87%. Its PXRD pattern indicate a phase mixture with main microcrystalline components identified as Ni3S2 and NiO (Fig. S13). The residual mass of the Co(II) sample of 25.16% at 975° C matches well with Co9S8 (theoretical 24.90%) as identified from the PXRD pattern.(Fig. S14).
The thermogravimetric heating curve for compound 3 is shown in Fig. 8. [Co(3-ampy)4(NCS)2] shows the following steps with weight losses: step 1: Δm = 34.25% (110–220 °C); step 2: Δm = 34.17% (220–470 °C); step 3: Δm = 15.25% (470–975 °C). The first two steps of weight loss are accompanied with sharp DSC signals at 195.2 °C and 341.3 °C, respectively. The first step of weight loss is accompanied by color change from orange to intensive blue and PXRD pattern of sample separated at 220 °C is identical with that of [Co(3-ampy)2(NCS)2] (4), (Fig. S15) confirming the release of two 3-ampy molecules (theoretical 34.13%). The weight loss of second decomposition step indicates further release of remaining two 3-ampy molecules to form intermediate Co(NCS)2, followed by further decomposition to Co9S8 at 975 °C as confirmed by PXRD (Fig. S16). (residual mass: 16.33%, theoretical mass: 15.31%).
Conclusions
In this work, three coordination polymers are formed through the interaction of metal(II) salts with 3-ampy as a co-ligand namely two 1D systems catena-{[Ni(μ2-3-ampy)(H2O)4]SO4·H2O} (1) and catena-{[Co(μ2-3-ampy)(H2O)4]SO4·H2O} (2), in addition to the expected monomeric species [Co(3-ampy)4(NCS)2] (3), [Co(3-ampy)2(NCS)2] (4), [Co(3-ampy)4(N3)2] (5) and mer-[Co(3-ampy)3(N3)3] (6), where in the latter case Co(II) was oxidized to Co(III). The isolation of the monomeric complexes through the coordination of the most basic pyridine-N-nitrogen is highly predictable. However, the 3-ampy molecule does behave as an “innocent” ligand as it looks. Surprisingly, it is the less basic amino group has very high tendency to coordinate to other metal ion and propagate the formation of polymeric chains with different dimensionality, as this was obvious in complexes 1 and 2.
In fact, the formation of CPs with 3-ampy reflects the general trend of this molecule to act as a bridging ligand through its two N-atoms. This trend was demonstrated by the isolation of many CPs with different metal ions such as Cd(II), Cu(II), Ni(II), Co(II), Ag(I) and Ag(II) but still the highest majority were obtained with Cd(II) (Table 1). Pseudohalides used here and or those reported in Table 2 behaved as simple terminal monodentate ligands. Other carboxylate (acetate, benzoate, isophthalate, adipate) or oxyanions (nitrate, sulfonylate,…) seem to be more effective as bridging ligands in the presence of 3-ampy in expanding the network chains (Table 2). Another interesting feature is provided by the 3-ampy co-ligand in its complexes is its ability to form supramolecular hydrogen bonded systems via the NH2-group and non-covalent π–π ring–ring interactions via its pyridine moiety, which adds extra stability to the resulted compounds.
Thermal analysis confirms that [Co(3-ampy)2(NCS)2] (4) can be obtained at 220 °C by release of two 3-ampy molecules from [Co(3-ampy)4(NCS)2] (3) starting material. Co9S8 is obtained as final decomposition product (975 °C, N2 atmosphere) in case of Co(II) compounds 2 and 3.
Supplementary data
CCDC 1982388-1982393 contains the supplementary crystallographic data for 1–6, respectively. These data can be obtained free of charge via http//www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+ 44) 1223-336-033; or e-mail: deposit@ccdc cam.ac.uk. Further supplementary data: bond parameters (Tables S1-S3), hydrogen bond systems (Table S4), non-covalent ring-ring interactions (Table S5); packing plots (Figs. S1-S6), XRD powder pattern (Figs. S7-S12), for compounds 1–6, respectively; XRD powder pattern (Figs. S13-S16) for decomposition products of 1–3, respectively.
References
Mautner FA, Jantscher P, Fischer RC, Torvisco A, Vicente R, Karsili TNV, Massoud SS (2019) Polyhedron 166:36–43
Goher MAS, Hafez AK, Abu-Youssef MAM, Badr AMA, Gspan C, Mautner FA (2004) Polyhedron 23:2349–2356
Mautner FA, Goher MAS, Moustafa HE, Abu-Youssef MAM, Öhrström N (2007) Polyhedron 26:2703–2712
Soliman SM, Elsilk SE (2017) J Mol Struct 1149:58–68
O’Donovan ME, LaDuca RL (2014) J Mol Struct 1070:21–27
Zheng X-F, Zhu L-G (2011) Z Anorg Allg Chem 637:1566–1572
Abu-Youssef MAM, Langer V, Öhrström L (2006) Chem Commun 10:1082–1084
Meng Y-T, Ruan Y-J, Mo J-P, Liu B-L, Wang A-Y, Xiao H-P (2017) Trans Met Chem 42:285–291
Shen Y, Ma N, Wu L, Song H-H (2015) Inorg Chim Acta 429:51–60
Dojer B, Pevec A, Jagodic M, Kristl M, Drofenik M (2012) Inorg Chim Acta 383:98–104
Dojer B, Golobic A, Jaglicic Z, Kristl M, Drofenik M (2012) Monatsh Chem 143:73–78
Pan W-L, Huang K-L, Xu Y-Q, Hu C-W (2007) Chin J Struct Chem 26:822
He X, Wei Y-Q, Li M-X (2008) Chin J Struct Chem 27:1451
Yeh C-W, Jou C-H, Tsou C-H, Suen M-C (2012) Acta Crystallogr Sect E Struct Rep Online 68:m1204–m1205
Lah N, Clérac R (2009) Polyhedron 28:2466–2472
Moore MH, Nassimbeni LR, Niven ML (1987) Inorg Chim Acta 132:61–66
He H-Y, Zhu L-G (2006) Acta Crystallogr Sect E Struct Rep Online 62(m198):m200
Costes J-P, Novitchi G, Shova S, Dahan F, Donnadieu B, Tuchagues J-P (2004) Inorg Chem 43:7792–7799
Castillo O, Luque A, Roman P, Lloret F, Julve M (2001) Inorg Chem 40:5526–5535
Dutta D, Nashreul-Islam MS, Saha U, Chetry S, Guha AK, Bhattacharyya MK (2018) J Chem Cryst 48:156–163
Bucar D-K, Mestrovic E (2003) Acta Crystallogr Sect E: Struct Rep Online 59:m985–m987
Youssef MA, Escuer A, Goher MAS, Mautner FA, Vicente R (2000) J Chem Soc Dalton Trans 413:413–416
Pandey P, Kharediya B, Elrez B, Sutter J-P, Bhargavi G, Rajasekharan MV, Sunkari SS (2017) Dalton Trans 46:15908–15918
Arumuganathan T, Rao AS, Das SK (2010) Cryst Growth Des 10:4272–4284
Izumi HK, Kirsch JE, Stern CL, Poeppelmeier KR (2005) Inorg Chem 44:884–895
Lah N, Leban I (2005) Acta Crystallogr Sect E Struct Rep Online 61:m1708–m1710
Fang X, Yang F-J, Yu H-Y, Chen N-S, Huang M-D, Wang J-D (2009) Inorg Chem Commun 12:664–666
Mautner FA, Berger C, Gspan C, Study B, Fischer RC, Massoud SS (2017) Polyhedron 130:136–144
Abu-Youssef MAM, Escuer A, Langer V (2006) Eur J Inorg Chem 16:3177–3184
Todd AM, Swinburne AN, Goeta AE, Steed JW (2013) New J Chem 37:89–96
Fomina I, Dobrokhotova Z, Aleksandrov G, Bogomyakov A, Fedin M, Dolganov A, Magdesieva T, Novotortsev V, Eremenko I (2010) Polyhedron 29:1734–1746
Wu J-Y, Feng D-M, He H-Y, Wang Q-X, Zhu L-G (2005) Acta Crystallogr Sect E Struct Rep Online 61:m1779–m1781
He H-Y, Zhu L-G (2003) Acta Crystallogr Sect E Struct Rep Online 59:m1192–m1193
Kartal Z, Sahin O, Yavuz A (2018) J Mol Struct 1171:578–586
Saha S, Kottalanka RK, Bhowmik P, Jana S, Harms K, Panda TK, Chattopadhyay S, Nayek HP (2014) J Mol Struct 1061:26–31
Kojima T, Nakanishi T, Honda T, Harada R, Shiro M, Fukuzumi S (2009) Eur J Inorg Chem 6:727–734
Willett RD, Halvorson K (1988) Acta Crystallogr Sect C Cryst Struct Commun 44:2068–2071
Ilyukhin AB, Koroteev PS, Novotortsev VM (2019) J Mol Struct 1187:38–49
Medved’ko AV, Churakov AV, Yu H, Li W, Vatsadze SZ (2017) Acta Crystallogr. Sect E Cryst Commun 73:856–858
Peng Z-S, Zhang C-L, Shen X-M, Deng Q, Cai T-J (2011) J Coord Chem 64:2848–2858
Roman P, Luque A, Beitia JI, Guzman-Miralles C (1992) Polyhedron 11:1883–1890
Deng Q, Huang Y, Peng Z, Dai Z, Lin M, Cai T (2013) J Solid State Chem 200:60–69
Cherif I, Zid MF, El-Ghozzi M, Avignant D (2012) Acta Crystallogr Sect E Struct Rep Online 68:m900–m901
Willett RD, Place H, Middleton M (1988) J Am Chem Soc 110:8639–8650
Blanchette JT, Willett RD (1988) InorgChem 27:843–849
Ali BF, Al-Far RH, Haddad SF (2008) Acta Crystallogr Sect E Struct Rep Online 64:m751–m752
Bruker (2005) SAINT v. 7.23; Bruker (2006) APEX 2, v. 2.0–2, Bruker AXS Inc., Madison, Wisconsin, USA, 2005
Sheldrick GM (2001) SADABS v. 2, University of Goettingen, Germany
Sheldrick GM (2008) Acta Crystallogr, A 64:112–122
Sheldrick GM (2015) Acta Crystallogr, C 71:3–8
Macrae CF, Edington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler T, Van de Streek J (2006) J Appl Cryst 39:453–457
Speck AL (2001) PLATON, a multipurpose crystallographic tool. Utrecht University, Utrecht The Netherlands
V.A. Blatov, A.P. Shevchenko, D.M. Proserpio. ToposPro: Applied topological analysis of crystal structures with the program ToposPro (2014) Cryst Growth Des, 14: 3576–3586. (v. 5.4.0.2, realease date: 21.04.2020)
Nakamoto K (1978) Infrared spectra of inorganic and coordination compounds, 2nd edn. Wiley, New York
Henderson B, Imbusch GF (1989) Optical spectroscopy of inorganic solids. Oxford Science Publications, Oxford
Racah G (1942) Phy Rev 62:438–462
Tanabe Y, Sugano S (1954) J Phy Soc Japan 9:753–765
Housecroft CE, Sharpe AG (2012) Inorganic chemistry, 4th edn. Pearson Publisher, Harlow, England, p 691
Acknowledgements
F.A.M. thanks Anna Huber and K. Gatterer (TU Graz) for assistance and NAWI Graz for partial financial support.
Funding
Open access funding provided by Graz University of Technology..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mautner, F.A., Jantscher, P.V., Fischer, R.C. et al. Syntheses, structural characterization, and thermal behaviour of metal complexes with 3-aminopyridine as co-ligands. Transit Met Chem 46, 191–200 (2021). https://doi.org/10.1007/s11243-020-00436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00436-2