Abstract
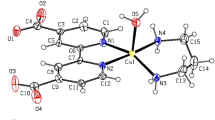
Two oxalato-bridged copper(II) complexes of formula, [Cu2(L1)2(µ-ox)](NO3)2·H2O, 1 and [Cu2(L2)2(µ-ox)](NO3)2·H2O, 2 (ox = oxalato dianion, L1 = N,N-dimethyl,N′-benzylethane-1,2-diamine, L2 = N,N-diethyl,N′-benzylethane-1,2-diamine), have been synthesized and characterized by elemental analyses, spectroscopic (IR, UV–Vis) data and molar conductance measurements. The crystal structure of complex 1 was determined by X-ray diffraction analysis, revealing two centrosymmetric dinuclear units. The first consists of a [Cu2(L1)2(µ-ox)(NO3)2] molecule, in which each Cu(II) center is in a square-pyramidal environment, providing two nitrogen atoms from the diamine-chelating ligands plus two oxygen atoms from the oxalate in the basal plane and an oxygen of the nitrate group in the axial position. The second unit [Cu2(L1)2(µ-ox)(H2O)2](NO3)2 has a similar structure, but the apical sites are occupied by water ligands and the nitrate anions are free from coordination. Both complexes are solvatochromic. Their solvatochromism was investigated with different solvent parameter models using SPSS/PC and DFT methodology. The solvatochromic behaviors of the complexes were also explored by TD-DFT in ethanol and acetonitrile solvents. The calculated visible absorption spectra were in accord with the experimental results.









Similar content being viewed by others
References
Fukuda Y (2007) Inorganic Chromotropism Basic Concepts and Applications of Colored Materials. Kodansha, Tokyo and Springer
Sone K, Fukuda Y (1983) Ions and Molecules in Solution. Elsevier, Amsterdam
Golchoubian H, Rezaee E, Bruno G, Rudbari HA (2011) Inorg Chim Acta 366:290–297
Golchoubian H, Rezaee E, Bruno G, Rudbari HA (2013) J Coord Chem 66:2250–2263
Asadi H, Golchoubian H, Welter R (2005) J Mol Struct 779:30–37
Golchoubian H (2008) Anal Sci 24:X169–X170
COSMO (2005) Bruker AXS Inc. Madison, Wisconsin
SAINT (2005) version 7.06A, Bruker AXS Inc, Madison, Wisconsin
SADABS (2005) version 2.10, Bruker AXS Inc, Madison, Wisconsin
Burla MC, Caliandro R, Camalli M, Carrozzini B, Cascarano GL, De Caro L, Giacovazzo C, Polidori G, Spagna R (2005) J Appl Crystallogr 38:381–388
Sheldrick GM (2015) Acta Cryst (2015) C71, 3–8
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Shida MI, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian Inc, Wallingford
Dennington R, Keith T, Millam J (2009) GaussView, Version 5. Semichem Inc., Shawnee Mission KS
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Yanai T, Tew D, Handy N (2004) Chem Phys Lett 393:51–57
Machura B, Małecki JG, witlicka AS, Nawrot I, Kruszynski R (2011) Polyhedron 30:864–872
Stratmann RE, Scuseria GE, Frisch MJ (1998) J Chem Phys 109:8218–8224
Ray S, Konar S, Jana A, Das K, Butcher RJ, Mondal TK, Kar SK (2013) Polyhedron 50:51–58
Barone V, Cossi M (1998) J Phys Chem A 102:1995–2001
Cossi M, Rega N, Scalmani G, Barone V (2003) J Comput Chem 24:669–681
Marcus Y (1993) Chem Soc Rev 22:409–416
Bag B, Mondal N, Mitra S, Gramlich V, Ribas J, El Fallah MS (2001) Polyhedron 20:2113–2116
Castillo O, Luque A, Julve M, Lloret F, Román P (2001) Inorg Chim Acta 315:9–17
Mautner FA, Louka FR, Massoud SS (2009) J Mol Struct 921:333–340
Golchoubian H, Rezaee E (2009) J Mol Struct 927:91–95
Castillo O, Muga I, Luque A, Gutiérrez-Zorrilla JM, Sertucha J, Vitoria P, Román P (1999) Polyhedron 18:1235–1245
Raman N, Esthar S, Thangaraja C (2004) J Chem Sci 116:209–213
Geary W (1971) J Coord Chem Rev 7:81–122
Addison AW, Rao TN, Reedijk J, Van Rijn J, Verschoor GC (1984) J Chem Soc, Dalton Trans 7:1349–1356
Andrade SG, Gonçalves LCS, Jorge FE (2008) J Mol Struct THEOCHEM 864:20–25
Tamer Ö, Avcı D, Atalay Y (2014) Spectrochim Acta A 117:78–86
Exner O (1988) In correlation analysis of chemical data. Plenum Press, New York
Acevedo-Martınez J, Escalona-Arranz JC, Villar-Rojas A, Tellez-Palmero F, Perez-Roses R, Gonzalez L, Carrasco-Velar R (2006) J Chromatogr A 1102:238–244
Acknowledgements
We are grateful for the financial support of the University of Mazandaran of the Islamic Republic of Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Samimi, R., Golchoubian, H. Dinuclear copper(II) complexes with ethylenediamine derivative and bridging oxalato ligands: solvatochromism and density functional theory studies. Transit Met Chem 42, 643–653 (2017). https://doi.org/10.1007/s11243-017-0170-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-017-0170-8