Abstract
Based on 5-mercapto-1H-tetrazole-1-methanesulfonic acid disodium salt (Na2mtms) and 4,4′-bipyridine (bpy) as ligands, four new transition metal complexes, namely {[Cd2(mtms)(bpy)2(OAc)2]·H2O} n (1), {[Cd(mtms)(bpy)2(H2O)2]2·bpy·4H2O} n (2), {[Zn2(μ 2-OH)(mtms)(bpy)3(H2O)]·ClO4·H2O} n (3), and {[Co(mtms)2(bpy)(H2O)2]·[Co(bpy)2(H2O)4]·H2O} n (4), have been synthesized and characterized by single-crystal X-ray diffraction. Complex 1 features a pillared-layer coordination architecture linked by acetate, mtms, and bridging bpy ligands. Complex 2 has a 1D polymeric structure with [Cd(mtms)(bpy)2(H2O)2] as the repeating unit; these infinite chains are further connected into a 3D supramolecular framework through π–π stacking of bpy ligands. In complex 3, the mtms ligand combined with μ 2-OH bridges two Zn atoms to form a dimer structure, which is different from that of complex 2. Complex 4 shows a 3D supramolecular network containing infinite [Co(mtms)2(bpy)(H2O)2]2− anionic chains and free [Co(bpy)2(H2O)4]2+ cationic components. The luminescence properties of 1 and 2 and the electrochemical properties of 3 are reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical templates have an important role in the formation of complexes containing multiple components that have defined structural assemblies [1, 2]. Several previous studies have shown that geometries and sizes of ligands, the coordinating ability of anions, and the nature of metals are all important in determining the structures of such complexes [3–8]. N-heterocycles with sulfhydryl ligands are capable of –N(H)–C(=S) ↔ –N=C(–SH) tautomerization. Heterocyclic nitrogen can participate in coordination, while the sulfhydryl moiety can be used as a bridging group [8–14]. The soft sulfhydryl group has good coordination ability, because it has empty 3d orbital that can accept electrons from the metal [8, 14–16]. Recently, research into nitrogen heterocycles with sulfhydryl ligands has mainly concentrated on sulfhydryl imidazole, sulfhydryl-(1,2,4)-triazole, and sulfhydryl tetrazole [8, 14, 17–19]. In our previous studies, we have reported some novel coordination polymers with 5-mercapto-1H-tetrazole-1-acetic acid [17]. In addition, bis- or multidentate ligands based on bipyridine (bpy) are outstanding bridging ligands, and a number of frameworks have been synthesized [20, 21]. Normally, 4,4′-bpy is an effective rodlike linker joining metal atoms for propagation of coordination networks [14, 21, 22].
Herein, we present four transition coordination polymers containing mtms (Scheme 1) and bpy (Scheme 2) co-ligands, namely {[Cd2(mtms)(bpy)2(OAc)2]·H2O} n (1), {[Cd(mtms)(bpy)2(H2O)2]2·bpy·4H2O} n (2), {[Zn2(μ 2-OH)(mtms)(bpy)3(H2O)]·ClO4·H2O} n (3), and {[Co(mtms)2(bpy)(H2O)2]·[Co(bpy)2(H2O)4]·H2O} n (4).
Experimental
Materials and measurements
All reagents were commercially available and used without further purification. Elemental analyses for C, H, N, and S were carried out with a Perkin-Elmer 2400II elemental analyzer. IR spectra were recorded on a Perkin-Elmer Spectrum One FTIR spectrometer with KBr pellets in the range 4,000–400 cm−1. Electrochemical measurements were made at room temperature using a BAS Epsilon electrochemical workstation with a conventional three-electrode cell consisting of a glassy carbon working electrode, a platinum auxiliary, and a saturated calomel reference electrode (SCE). KCl (0.1 mol L−1) was used as a supporting electrolyte in aqueous solution.
Synthesis of complex 1
Bipyridine (77.8 mg, 0.5 mmol) was added to a stirred solution of Na2mtms (120.6 mg, 0.5 mmol) and Cd(OAc)2·2H2O (274.0 mg, 1 mmol) in a mixture of 15 ml (1:1) H2O/EtOH. The mixture was stirred under reflux for 3 h and then filtered. After a month, colorless block crystals were obtained from the filtrate. Yield: 60 %. Anal. Calc. for C26H26Cd2N8O8S2 (%): C, 36.0; H, 3.2; N, 12.9; S, 7.4. Found (%): C, 36.0; H, 3.4; N, 12.7; S, 7.2. IR (KBr, cm−1): 3,432(w), 1,603(w), 1,567(w), 1,414(w), 1,288(s), 1,220(w), 1,183(m), 1,075(m), 1,046(w), 1,007(s), 807(m), 630(m).
Synthesis of complex 2
The synthesis of colorless block crystals of 2 followed the same procedure as for 1, except that Cd(OAc)2·2H2O was replaced by Cd(ClO4)2·6H2O. Yield: 50 %. Anal. Calc. for C54H60Cd2N18O14S4 (%): C, 42.2; H, 3.9; N, 13.7; S, 8.3. Found (%): C, 42.3; H, 3.8; N, 13.4; S, 8.5. IR (KBr, cm−1): 3,391(m), 1,601(w), 1,532(s), 1,490(s), 1,411(w), 1,399(m), 1,371(s), 1,355(m), 1,271(w), 1,249(m), 1,226(w), 1,180(w), 1,047(w), 1,006(s), 806(w), 629(m).
Synthesis of complex 3
The preparation of 3 was similar to 1, but using Na2mtms (120.6 mg, 0.5 mmol), Zn(ClO4)2·6H2O (372.4 mg, 1 mmol), H2O (10 mL), CH3OH (5 mL), and bpy (155.6 mg, 1 mmol). After 3 days, pale yellow block crystals were obtained. Yield: 55 %. Anal. Calc. for C33H35ClN10O11S2 Zn2 (%): C, 40.5; H, 3.6; N, 14.3; S, 6.6. Found (%): C, 40.4; H, 3.9; N, 14.2; S, 6.4. IR (KBr, cm−1): 3,427(w), 1,611(w), 1,596(w), 1,535(m), 1,489(s), 1,416(w), 1,389(m), 1,319(s), 1,216(w), 1,187(w), 1,105(w), 1,046(w), 1,016(m), 807(w), 642(m).
Synthesis of complex 4
Complex 4 was prepared by a similar procedure as described for 1, using Na2mtms (120.6 mg, 0.5 mmol), Co(NO3)2·6H2O (145.5 mg, 0.5 mmol), bpy (155.5 mg, 1 mmol), 15 ml (2:1) H2O/EtOH, and five drops of DMF. Red block crystals were obtained after 14 days. Yield: 61 %. Anal. Calc. for C34H48Co2N14O16S4 (%): C, 35.4; H, 4.2; N, 17.0; S, 11.1. Found (%): C, 35.1; H, 4.2; N, 17.1; S, 11.4. IR (KBr, cm−1): 3,411(w), 1,656(m), 1,629(m), 1,535(s), 1,492(s), 1,413(w), 1,390(s), 1,373(m), 1,293(m), 1,259(s), 1,238(w), 1,189(w), 1,068(m), 1,043(w), 813(m), 629(m).
X-ray crystallography
Diffraction data were collected on a Bruker Smart CCD diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at 298 K. The structures were solved by direct methods and refined by full-matrix least squares on F 2 using SHELXL-97 [23]. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were positioned geometrically and refined by a riding mode. The crystallographic data for 1–4 are listed in Table 1. Selected bond lengths and angles are listed in Table 2† in the Supplementary Information.
Results and discussion
Crystal structure of complex 1
As shown in Fig. 1a, complex 1 exhibits a dimeric structure in which two Cd atoms are bridged by two acetates; one adopts syn–syn mode (μ2, η2-carboxylato), and the other coordinates through one O atom (μ2, η1-carboxylato), which is different to other previously reported binuclear Cd(II) complexes [24]. The Cd···Cd distance is 3.9462 Å. Cd1 and Cd2 adopt octahedral coordination geometries. Cd1 is coordinated by three O atoms from two acetates, and three N atoms from two bpy ligands and one mtms ligand. Cd2 is ligated by two N atoms from two bpy ligands, two O atoms from two acetate anions, plus one O atom and one S atom from an mtms ligand. The mtms acts as a tridentate-bridging ligand (Scheme 1, mode I) to link adjacent dimeric Cd(II) units, generating a 1D zigzag infinite chain. The adjacent Cd-mtms chains are further connected into a 2D pillared-layer architecture by bridging-bpy ligands along the ac plane (Scheme 2, mode I) (Fig. 1b). Finally, complex 1 forms a supramolecular 3D network through intermolecular hydrogen bonds [O8–8C···O6 = 2.848(16) Å, O8–H8B···O2A = 2.788(19) Å] (symmetry code: A: x + 1/2, −y+3/2, z + 1/2) (Fig. 1c†). The relevant hydrogen bond parameters are summarized in Table 3†.
Crystal structure of complex 2
Complex 2 was synthesized in a similar way as for 1, except that Cd(OAc)2·2H2O was replaced by Cd(ClO4)2·6H2O. However, complex 2 shows a linear, infinite 1D polymeric structure with a neutral component [Cd(mtms)(bpy)2(H2O)2] as the repeating unit (Fig. 2a), which is quite different from complex 1. The Cd atom is in a distorted octahedral geometry, with three N atoms from three bpy ligands, two O atoms from two coordinated water ligands, and one S atom from an mtms ligand. The mtms ligand adopts an S-bonding monodentate coordination mode (Scheme 1, mode II), and the tetrazole ring is on the outside, as also found in our previously reported mercapto-tetrazole complexes [Cd(mtz)2Br2] (mtz = 1-[2-(N,N-dimethylamino-ethyl]-5-mercapto-1H-tetrazole) [8] and {[CoIII(mmtz)2(1,10-phen)2]·NO3} (mmtz = 1-methyl-5-mercapto-tetrazole [14]. Of the bpy ligands in complex 2, one adopts a terminal coordination mode to link Cd(II) forming [CdN3O2S] units (Scheme 2, mode II), while the other acts as a spacer to bridge the [CdN3O2S] units, leading to 1D infinite chains along the b axis (Scheme 2, mode I). These 1D chains are further connected into a 3D supramolecular framework through π–π stacking interactions from the free neutral bpy ligands (Scheme 2, mode V) and hydrogen bonds among the bpy ligands (Scheme 2, mode Ш, IV), coordinated water, and the uncoordinated O atom of the mtms ligand (Fig. 2b†). In short, the bpy ligands play an important role in forming the structure of 2, revealing five different coordination modes.
Crystal structure of complex 3
Use of Zn(ClO4)2·6H2O instead of Cd(ClO4)2·6H2O gave complex 3, whose structure with P2(1)/c space group is different from that of complex 2. In complex 3 (Fig. 3a), the bidentate-bridging mtms ligand (Scheme 1, mode III) combines with an μ 2-OH bridge between two Zn atoms to form a binuclear unit. The coordination environment of 3 shows a butterfly-like structure, in which four bpy ligands act as the wings, while the mtms ligand is located at the head. Zn1 is surrounded by two bpy ligands, one mtms ligand, one coordinated water ligand, and one hydroxy ligand to form a distorted trigonal–bipyramidal geometry. The axial positions are occupied by N4 and O5 with an N4–Zn1–O5 bond angle of 174.12(13)°. While Zn2 is in a distorted tetrahedral coordination geometry provided by one hydroxy, one mtms, and two bpy ligands. The Zn–N bond distances range from 2.022(4) to 2.215(4) Å, and the Zn–S bond distance is 2.3215(16) Å, which are similar to other complexes [23, 24]. The binuclear units link each other by bpy ligands (Scheme 2, mode I, II) to form a 1D zigzag chain along the c axis. The most important interchain interaction in the crystal packing is hydrogen bonds among uncoordinated ClO4 −, coordinated hydroxy, coordinated water, free water, the uncoordinated sulfonic group of mtms, and the bpy ligand (Scheme 2, mode III) (Fig. 3b†). Compared to the similar coordination modes of bpy in complexes 2 and 3, the architecture of 3 is more complicated. The tetrazolyl-N of mtms can provide potential coordination sites, and uncoordinated ClO4 − anion may also help to determine the final coordination architectures.
Crystal structure of complex 4
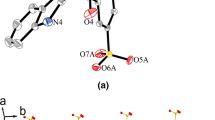
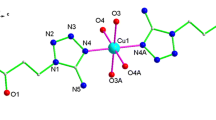
As illustrated in Fig. 4a, complex 4 contains two components, namely [Co(mtms)2(bpy)(H2O)2]2− and [Co(bpy)2(H2O)4]2+ ions. Atom Co1 is located on a crystallographic inversion center and octahedrally coordinated by two mtms N atoms and two bpy N atoms in the equatorial plane, plus two coordinated water ligands in the axial positions, with an O4–Co1–O4A bond angle of 180.0°. The coordination environment of Co2 is provided by two bpy N atoms in the axial positions, with four coordinated water ligands in the equatorial plane. The Co2–N bond distances range from 2.179(6) to 2.203(5) Å, which are comparable with other complexes [25].
a View of the coordination environment of the Co(II) center in 4. Symmetry codes: A: −x + 1, −y + 1, −z; B: −x + 2, −y, −z + 1. b View of the 1D infinite chain of Co1 along the b axis. c Stacked plot of 4 with the 1D chains of Co1 and free Co2 units. d A schematic illustration of the packing of 4. Symmetry codes: (A) –x + 1, −y + 1, −z; (B) x, y − 1, z; (C) –x + 2, −y, −z; (D) x + 1, y − 1, z; (E) x + 1, y, z; (F) –x + 1, −y + 1, −z + 1; (G) x, y + 1, z; (H) – x + 1, −y + 2, −z + 1
The structure of the complex anion [Co(mtms)2(bpy)(H2O)2]2− is the same as that of our previously reported complex [CoII(mmtz)2(4,4′-bpy)(H2O)2] n (mmtz = 1-methyl-5-mercapto-tetrazole) [14]. The mtms acts as a N-bonding monodentate ligand (Scheme 1, mode IV). The bpy spacers bridge adjacent Co1 atoms to generate a 1D chain along the b axis (Scheme 2, mode II). The bpy ligands adopt terminal coordination mode (Scheme 2, mode I) to link Co2 centers, forming the cationic complex [Co(bpy)2(H2O)4]2+. These Co2 components are vertically penetrating into the 1D chains of Co1, showing a rail-like structure (Fig. 4d). The supramolecular structure mainly depends on the π–π stacking of bpy (Scheme 2, mode V), and intermolecular hydrogen bonds among the water ligands, the uncoordinated sulfonic group of the mtms ligand, and the bpy ligand (Scheme 2, mode III).
Photoluminescence properties
It is well known that Cd(II) complexes can exhibit interesting luminescence properties [8, 24]. The solid-state luminescence of the free Na2mtms ligand and complexes 1 and 2 was investigated at room temperature and is shown in Fig. 5†. Na2mtms shows an emission peak at 460 nm when excited at 301 nm. Upon excitation at 304 nm, complexes 1 and 2 display emission maxima at 353 and 362 nm, respectively, which are both greatly blue-shifted compared to the free Na2mtms ligand. Since the Cd(II) center has d 10 electronic configuration, the emissions of complexes 1 and 2 can be tentatively assigned to ligand-to-metal charge transfer (LMCT) [26–28]. These complexes may be suitable as blue-light-emitting materials, since they show luminescence in the blue region [29].
Cyclic voltammetry
The cyclic voltammogram of 3 was measured with a conventional three-electrode cell in aqueous solution with complex concentration of 1.0 × 10−5 mol L−1, as shown in Fig. 6†. Measurements were carried out at room temperature by scanning from −0.600 to +1.400 V at different scan rates. The CV shows an oxidation peak at 0.444 V with the scan rate of 50 mV s−1, while there is no corresponding reduction wave. As the scan rate is increased to 80 and 100 mV s−1, the anodic peak appears at 0.479 and 0.567 V, respectively. Again, no corresponding cathodic wave was observed, showing that the electrochemical behavior of the Zn(II)/Zn(I) redox couple is irreversible [30].
Conclusion
Four new complexes have been constructed from mixed mtms and bpy ligands. The 2D pillared-layer structure of 1 is obtained with carboxylato groups, while complexes 2, 3, and the host structure 4 all exhibit 1D infinite chain structures. The results demonstrate that coordinated anions can increase the dimensionality of the crystal structures, while uncoordinated anions can propagate the architectures through hydrogen bonding and π–π stacking interactions. The mtms ligand shows four different coordination modes in the four complexes. The varied coordination modes of the neutral rigid linear bpy ligand (bridging coordination, terminal coordination, hydrogen bond, and π–π stacking) found in this study proves that it is an excellent building block in constructing novel hybrid frameworks. In summary, for the complexes 1–4 described in this paper, we can see that the properties of the anion, mtms, and bpy ligands all help to determine the final coordination architectures.
Supplementary Information
CCDC 929454–929457 contain the supplementary crystallographic data for complexes 1–4, respectively. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Anderson S, Anderson HL, Sanders JKM (1993) Acc Chem Res 26:469–475
Beer PD, Gale PA (2001) Angew Chem 113:502–532
Tseng TW, Luo TT, Chen CC, Su CC, Chi KM, Lu KL (2013) Cryst Growth Des 13:510–517
Yin PX, Zhang J, Li ZJ, Qin YY, Cheng JK, Zhang L, Lin QP, Yao YG (2009) Cryst Growth Des 9:4884–4896
Lassig D, Lincke J, Moellmer J, Reichenbach C, Moeller A, Glaser R, Kalies G, Cychosz KA, Thommes M, Staudt R, Krautscheid H (2011) Angew Chem Int Ed 50:10344–10348
Zheng ST, Bu JT, Li YF, Wu T, Zuo F, Feng PY, Bu XH (2010) J Am Chem Soc 132:17062–17064
Millange F, Medina MI, Guillou N, Férey G, Golden KM, Walton RI (2010) Angew Chem Int Ed 49:763–766
Hao HM, Huang FP, Bian HD, Yu Q, Sun XL, Liang H (2011) Polyhedron 30:2099–2105
Matyáš R, Pachman J (2013) Primary explosives. Springer, Berlin, pp 227–254
Erkhitueva EB, Egorov DM, Dogadina AV, Khramchikhin AV, Ionin BI (2012) J Gen Chem 82(12):2011–2012
Akrivos PD (2001) Coord Chem Rev 213:181–210
Tamilselvi A, Mugesh G (2011) Inorg Chem 50:749–756
Biswas N, Thomas S, Sarkar A, Mukherjee T, Kapoor S (2009) J Phys Chem C 113:7091–7100
Li Y, Wang CQ, Bian HD, Huang FP, Liang H, Yu Q (2012) J Coord Chem 65:3665–3673
Tamilselvi A, Mugesh G (2011) Inorg Chem 50:749–756
Biswas N, Thomas S, Sarkar A, Mukherjee T, Kapoor S (2009) J Phys Chem C 113:7091
Yu Q, Huang FP, Yang ZM, Jin J, Bian HD, Liang H (2012) Polyhedron 33:203–208
Klinowski J, Paz FAA, Silva P, Rocha J (2011) Dalton Trans 40:321
Mondal R, Basu T, Sadhukhan D, Chattopadhyay T, Bhunia M (2009) Cryst Growth Des 9:1095–1105
De Melo ACC, De Amorim IF, Cirqueira ML, Martins FT (2013) Cryst Growth Des 13:1558–1569
Huang FP, Yu Q, Bian HD, Yan SP, Liang H (2008) Polyhedron 27:3160–3166
Huang FP, Li HY, Tian JL, Gu W, Jiang LM, Yan SP, Liao DZ (2009) Cryst Growth Des 7:3191–3196
Sheldrick GM (1997) SHELXL-97, program for X-ray crystal structure refinement. GÖttingen University, Germany
Wang XJ, Liu YH, Xu CY, Hou HW, Fan YT (2012) Cryst Growth Des 12:2435–2444
Jun H (2005) J Mol Struct 752:166–169
Sun L, Ma L, Cai JB, Liang L, Deng H (2012) Cryst Eng Comm 14:890–898
Li Q, Fu ML, Liu X, Guo GC, Huang JS (2006) Inorg Chem Comm 9:767–771
Li XP, Zhang JY, Pan M, Zheng SR, Liu Y, Su CY (2007) Inorg Chem 46:4617
Gao JY, Wang N, Xiong XH, Chen CJ, Xie WP (2013) Cryst Eng Comm 15:3261–3270
Odabas Z, Kara H, Özkaya AR, Bulut M (2012) Polyhedron 39:38–47
Acknowledgments
We gratefully acknowledge the National Nature Science Foundation of China (Nos. 21101035 and 21061002), Guangxi Natural Science Foundation of China (2010GXNSFF013001, 2012GXNSFAA053035, and 2012GXNSFBA053017), the Foundation of Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (CMEMR2011-20), and the Nature Science Foundation of Guangxi Normal University.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Su, CH., Wang, YK., Huang, FP. et al. Four transition metal complexes constructed with mixed mercaptotetrazole and 4,4′-bipyridine ligands. Transition Met Chem 38, 757–763 (2013). https://doi.org/10.1007/s11243-013-9746-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-013-9746-0