Abstract
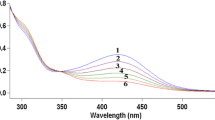
The kinetics of oxidation of N,N′-ethylenebis(isonitrosoacetyleacetoneimine)copper(II) complex, CuIIL, by N-bromosuccinimide (SBr) in weakly aqueous acidic solutions was studied under pseudo-first-order conditions. Plots of ln(A ∞ − A t ) versus time where A t and A ∞ are absorbance values of the Cu(III) product at time t and infinity, respectively, showed marked deviations from linearity. The curves showed an acceleration of reaction rate consistent with an autocatalytic behavior. In the presence of Hg(II) ions, plots of ln(A ∞ − A t ) versus time are linear up to >85 % of reaction. The value of the observed rate constant, k obs, increases with decreasing pH. At constant reaction conditions, the dependence of the observed rate constants, k obs, is described by Eq. (1).
The dependence of both k o and k 1 on [SBr] is not linear. The mechanism of the title reaction is consistent with an inner sphere mechanism in which a pre-equilibrium step precedes the electron transfer step. The overall rate law is represented by Eq. (2) where [CuIIL]t and K 1 represent the total copper(II) complex concentration and the pre-equilibrium formation constant, respectively.
.




Similar content being viewed by others
References
Levason W, Spicer MD (1987) Coord Chem Rev 76:45 and references therein
Margerum DW, Owens GD (1981) Met Ions Biol Syst 12:75
Sulfab Y, Al-Sogair FM (2002) Trans Met Chem 27:99
Sulfab Y, Al-Sogair FM (2004) Polyhedron 23:583
Bhula R, Weatherburn DC, Gainsford GJ (1987) Inorg Chim Acta 128:L7
Finar IL (1986) Organic Chemistry, vol 1, 6th edn. Longman, New York, p 452
Smith MB (2001) March J March’s Advanced Organic Chemistry; Reactions, Mechanisms and Structure, 5th edn. John Wiley and Sons, New York, p 911
Tanemura K, Suzuki T, Nishida Y, Satsumabayashi K, Horagushi T (2003) Chem Lett 32:932
Podgorsek A, Stavber S, Zupan M, Iskara J (2006) Tetrahedron Lett 47:1097
Tanemura K, Suzuki T, Nishida Y, Satsumabayashi K, Horagushi T, (2004) Chem. Commun. 470
Bertelsen S, Halland N, Bachmann S, Marigo M, Braunton A, Jorgensen KA (2005) Chem. Commun. 4821
Rahman ANMM, Bishop TR, Shan N (2005) Green Chem 7:207
Ahmed SA, Awad IMA, Abdel-Wahab AA (2002) Photochem Photobiol 1:84
Cope AC, Burrows EP, Derieg M, Moon S, Wirth WD (1965) J Am Chem Soc 87:5452
Abdel Khalek AA, Al-Obadie MS, Al-Mahdi AAK (1980) J Inorg Nucl Chem 42:1407
Kasuga K, Nagahara T, Yamamoto Y (1986) Inorg Chim Acta 113:13
Filler R (1963) Chem Rev 63:21
Kruse PF Jr, Grist KL, McCoy TA (1954) Anal Chem 26:1319
Lecomte J, Gault H (1954) Compt. Rend. 238:2538
Mathur NK, Narang CK (1975) The Determination of Organic Compounds with N-bromosuccininmide. Academic Press, New York
Abdel-Khalek AA, Sayyah EM, Khaled ESH (1993) Transition Met Chem 18:555
Abdel-Khalek AA, Khaled ESH (1998) Transition Met Chem 23:201
Abdel-Khalek AA, Khalil MM, Khaled ESH (1993) Transition Met Chem 18:153
Abdel-Khalek AA, Abdel-Hady AEA, El-Shahat MF (1993) Transition Met Chem 18:283
Abdel-Khalek AA, Sayyah EM, Khaled ESH (1998) Transition Met Chem 23:37
Abdel-Khalek AA, Khaled ESH (1999) Transition Met Chem 24:189
Abdel-Khalek AA, Abdel-Hady AEA, El-Shahat MF (1995) Transition Met Chem 20:430
Al-Shihri AM, Abdel-Hady AEA (1996) Transition Met Chem 21:406
Abdel-Khalek AA, Sayyah EM, Khaled ESH (1998) Indian J Chem 37A:489
Eljack ND, Sulfab Y (2012) Polyhedron 44:28
Eberson L, Barry JA, Finkelstein M, Moore WM, Ross SD (1986) Acta Chem Scand B 40:283
Elsammani HEA, Sulfab Y (2011) Transition Met Chem 36:271
Welcher FJ (1966) Organic analytical reagents, vol 3, 4th edn. D. Van Nostrand, New York, p 280
Aly MM, El-Said FA (1981) J Inorg Nucl Chem 43:287
Singh K, Singh V, Rahmani S, Singh AK, Singh B (2002) J Mol Catal A: Chem 197:91
Addison AW, Carpenter M, Lau LK, Wicholas M (1978) Inorg Chem 17:1545
Wilkins RG (2002) The study of kinetics and mechanism of reactions of transition metal complexes. Wiley-VCH Verlag GmbH & Co, Weinheim
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salih, I.M., Aljaber, A.S., Eltayeb, M.AZ. et al. Inner sphere oxidation of N,N′-ethylenebis(isonitrosoacetyleacetoneimine)copper(II) by N-bromosuccinimide in aqueous weakly acidic solutions. Transition Met Chem 38, 213–218 (2013). https://doi.org/10.1007/s11243-012-9680-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-012-9680-6