Abstract
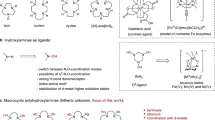
Mono-Schiff base complexes of Co(II) and Mn(III) with either benzo-10-aza-crown ether pendants (MnL 12 Cl, MnL 22 Cl) or morpholino pendants (MnL 32 Cl, CoL 32 ) have been employed as models for hydrolase enzymes by studying the kinetics of their hydrolysis reactions with p-nitrophenyl picolinate (PNPP). A kinetic model of PNPP cleavage catalyzed by these complexes is proposed. The effects of complex structures and reaction temperature on the rate of catalytic PNPP hydrolysis have also been examined. The rate increases with pH of the buffer solution; all four complexes exhibited high activity in the catalytic PNPP hydrolysis. Compared with the crown-free analogs MnL 32 Cl and CoL 32 , the crowned Schiff base complexes (MnL 12 Cl, MnL 22 Cl) exhibited higher catalytic activity. The pseudo-first-order rate constant for the PNPP hydrolysis catalyzed by the complex MnL 12 Cl, containing a benzo-10-aza-crown ether pendant, is 1.84 × 103-fold higher than that of spontaneous hydrolysis of PNPP at pH 7.60, 25 °C, [S] = 2.0 × 10−4 mol dm−3.
Graphical abstract
Four mono-Schiff base complexes of Mn(III) and Co(II) with either benzo-10-aza-crown ether pendants (MnL 12 Cl, MnL 22 Cl) or morpholino pendants (MnL 32 Cl, CoL 32 ) have been employed as models for hydrolase enzymes by studying the kinetics of their hydrolysis reactions with p-nitrophenyl picolinate (PNPP). The effects of ligand structure and reaction temperature on PNPP catalytic hydrolysis have been also discussed. All four complexes exhibited high activity in the catalytic PNPP hydrolysis. Compared with the crown-free analogy MnL 32 Cl and CoL 32 , the crowned Schiff base complexes MnL 12 Cl and MnL 22 Cl exhibit higher catalytic activity, the catalytic activity of the complexes follows the order Mn(III) > Co(II) under the same ligand.








Similar content being viewed by others
References
Rick LH, Judith NB (1998) Coord Chem Rev 173:133
Frasto da Silva JJR, Williams RJP (1994) The biological chemistry of the elements. Clarendon Press, Oxford
Cullis PM (1987) In: Page MI, Williams A (eds) Enzyme mechanism, Royal Society of Chemistry, London
Rawji GH, Yamada M, Sadler NP (2000) Inorg Chim Acta 303:168
Baykal U, Akkaya MS, Akkaya EU (1999) J Mol Catal A Chem 145:309
Ichikawa K, Tarnai M, Uddin MK (2002) J Inorg Biochem 91:437
Manseki K, Nakamura O, Horikawa K (2002) Inorg Chem Commun 5:56
Kou XM, Cheng SQ, Du J et al (2004) J Mol Catal A Chem 210:23
Cheng SQ, Wang YR, Zeng XC (2007) Colloids Surf A 292:32
Jiang WD, Xu B, Li JZ et al (2006) J Dispers Sci Technol 27:867
Li JZ, He XY, Wang Y et al (2011) Chin J Chem 29:1894
Zhang J, Tang Y, Li JZ et al (2009) Trans Met Chem 34:467
Tang Y, Li JZ, Zhang J et al (2009) Prog React Kinet Mech 34:373
Feng XS, Tang Y, Li JZ et al (2009) J Chem Res 6:345
Galan A, Andreu D, Echavarren AM et al (1992) J Am Chem Soc 114:1511
Li JZ, Feng FM, Xu B et al (2008) Trans Met Chem 33:655
Li JZ, Feng FM, Zhang J et al (2011) J Dispers Sci Technol 32:14
Sigman DS, Jorgensen CT (1972) J Am Chem Soc 94:1724
Li JZ, He XY, Zeng J et al (2012) Trans Met Chem 37:31
Li JZ, Wei L, Feng FM et al (2010) Trans Met Chem 35:463
Jiang WD, He XY, Wang Y et al (2011) Chin J Chem 29:2068
Lipscomb WN, Sträter N (1996) Chem Rev 96:2375
Li JZ, Jiang WD, Xu B et al (2009) Chin J Chem 27:372
Hettich R, Schneider HJ (1997) J Am Chem Soc 119:5638
Jiang FB, Jiang BY, Chen Y et al (2004) J Mol Catal A Chem 210:9
Liu Q, Chen HM, Lin H et al (2007) J Mol Catal A Chem 269:104
Kimura E, Hashinmoto H, Koike T (1996) J Am Chem Soc 118:10963
Fife TH, Przystas TJ (1985) J Am Chem Soc 107:1041
Acknowledgments
The authors gratefully acknowledge financial support from China National Natural Science Foundation (No.20072025), Key Scientific and Technological Project Issued by Ministry of Education of China (No. 208118), and the Opening Project of Key Laboratory of Green Catalysis of Sichuan Institutes of High Education (No. LZJ01, LZJ1101).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, Y., Zhang, J., Li, Jz. et al. Mono-Schiff base complexes with aza-crown ether or morpholino pendants as synthetic hydrolases for p-nitrophenyl picolinate cleavage. Transition Met Chem 38, 149–155 (2013). https://doi.org/10.1007/s11243-012-9672-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-012-9672-6