Abstract
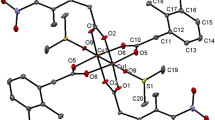
The monomeric CuII complex, [Cu(TLA)C2O4].2O (1), and dimeric CuII complex, [Cu2(TLA)2(C4O4)](ClO4)2 (1), where TLA=[tris(6-methyl-2-pyridyl)methyl]amine, have been synthesized and characterized. Both complexes have been obtained as single crystals and their structures were determined by X-ray diffraction analysis. The configuration of (1) is pseudo-octahedral owing to the fact that one of the Cu-Npyridyl bonds is much longer than the others. Two molecules of (1) link via hydrogen bonding to the oxalic oxygen in the unit cell. The copper complex (2) dimer is obtained, using the squarate ion as a bridging ligand, from a monomer copper complex, [Cu(TLA)N3].4ClO4 (3). By comparison of the configurations of (1), (2) with that of (3), the copper center is coordinated with TLA first, and then with secondary ligands, such as the azido group, squaric acid, or oxalate ion. The configurations of the transition metal complexes are affected by the smaller ions, as is further discussed below. The variable temperature magnetic susceptibilities of (2) have been studied, and the exchange integral is J=−2.5474 cm-, g=2.061, indicating the existence of a weaker anti-ferromagnetic interaction between the two copper ions in compound (2). The spectral behavior of the title complexes in solution has been studied by u.v.–vis. and i.r. techniques, and the results are discussed.
Similar content being viewed by others
References
C. Wendelstorf and R. Kramer, Angew. Chem., Int. Ed. Engl., 36, 2791 (1997).
M. Tsaramyrsi, M. Kaliva, A. Salfoglou, C. P. Raptopoulou, A. Terzic, V. Tangoulis and J. Giapintzakis, Inorg. Chem., 40, 5772 (2001).
H. Karosaki, H. Yoshida, A. Fujimoto, M. Goto, M. Shionoya, E. Kimura, E. Espinosa, J-M. Barbe and R. Guilard, J. Chem. Soc., Dalton. Trans., 898 (2001).
Z-H. Zhang, X-H. Bu, Z-H. Ma, W-M. Bu, Y. Tang and Q-H. Zhao, Polyhedron, 19, 1559 (2000).
Z-H. Zhang, H-L. Yang, Z-H. Ma and A-D. Qi, Chinese J. Chem., 18, 842 (2000).
Z-H. Zhang, X-H. Bu, Z-A. Zhu, Z-H. Jiang and Y. Chen, Transition Met. Chem., 21, 235 (1996).
G. R. Hay, J. C. Thibeault and R. Hoffmann, J. Am. Chem. Soc., 97, 4884 (1975).
M. Gupta, P. Mathur and R. J. Butcher, Inorg. Chem., 40, 878 (2001).
M. Kodera, K. Katayama, Y. Tachi, K. Kano, S. Hirota, S. Fujinami and M. Suzuki, J. Am. Chem. Soc., 121, 11006 (1999).
S. Itoh, H. Nakao, L. M. Berreau, T. Konso, M. Komatsu and S. Fukuzumi, J. Am. Chem. Soc., 120, 2890 (1998).
E. I. Solomon, M. J. Baldwin and M. D. Lowery, Chem. Rev., 92, 521 (1992).
M. M. De Mota, J. Rodgers and S. M. Nelson, J. Chem. Soc., A, 2036 (1969).
P. W. Selwood, Magnetochemistry, Interscience, New York, 1956, pp. 78–91.
Z. H. Zhang, Z. H. Ma, Y. Tang and W. J. Ruan, J. Chem. Crystallgr., 2, 119 (2004).
G. M. Sheldrick, SHELXTL 97 Programs for Crystal Structures, University of Göttingen, Germany, 1997.
B. Hathaway, Comprehensive Coordination Chemistry, Pergamon Press, Oxford, 1985, vol. 5.
J. Glerup, P. A. Goodson, D. J. Hodgson and K. Michelsen, Inorg. Chem., 34, 6255 (1995).
W-Z. Wang, X. Liu, Y. Meng, D-Z. Liao et al., Chem. Res. Chinese Univ., 1, 6 (2003).
C. Djordjevic, M. Lee and E. Sim, Inorg. Chem., 28, 719 (1989).
G. B. Deacon and R. Philips, J. Coord. Chem. Rev., 30, 227 (1980).
M. Tsaramyrsi, M. Kaliva, A. Salifoglou, C. P. Raptopoulou, A. Terzis, V. Tangorlis and J. Giapintzakis, Inorg. Chem., 40, 5772 (2001).
L. A. Hall, and D. J. Williams, in A. G. Sykes (Ed. ), Advances in Inorganic Chemistry, Including Bioinorganic Studies, Academic Press, vol. 52, New York, 2001.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th Edit., Wiley, New York, 1986.
F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, Comprehensive Text Book, 3rd Edit., Wiley, New York, 1972.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, Zh., Yang, Hl., Tang, Y. et al. The effect of small ions on transition metal complex configurations. Synthesis, crystal structures and properties of two new copper(II) complexes containing a tripodal ligand. Transition Metal Chemistry 29, 590–595 (2004). https://doi.org/10.1007/s11243-004-1033-7
Issue Date:
DOI: https://doi.org/10.1007/s11243-004-1033-7