Abstract
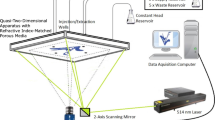
Visualising fluid flow in porous media using optical techniques is challenging due to the inability to see through the medium. Here, we present an experimental methodology based on shadowgraphy to investigate the dynamic spreading of convective plumes in saturated transparent porous media made of glass beads. The saturated porous medium can be tuned transparent by matching the refractive index of the solid glass beads to that of the saturating fluid mixture. The proposed technique allows to investigate the essential elements of convective mixing within a porous medium using miscible fluids. We also describe a method to determine the velocity of convective plumes as they propagate. Our experimental results show that the density difference achieved during convection significantly affects the convective front velocity of the plumes. This is significant because it allows to quantitatively predict the intensity of convective mixing in porous media from the speed of the convective front.
Article Highlights
-
Shadowgraphy has been successfully used to investigate fluid flow in transparent porous media.
-
Convective front velocities are used to evaluate quantitatively convective mixing in porous media.
-
In the fluid flow regime of our experiment, we observed a super-linear scaling of the convective front velocity and density difference.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Alipour, M., De Paoli, M., Soldati, A., Alipour, M., De Paoli, M., Soldati, A.: Concentration-based velocity reconstruction in convective Hele-Shaw flows. ExFl. 61(9), 195 (2020). https://doi.org/10.1007/S00348-020-03016-3
Almasi, M.: Molecular interactions and structural studies of toluene and (C5–C10) 1-alkanol; mutual diffusion and virial coefficients. J. Mol. Struct. (2021). https://doi.org/10.1016/j.molstruc.2020.129624
Appelo, C.A.J., Postma, D.: Geochemistry, Groundwater and Pollution. CRC Press, Boca Raton (2004)
Archie, G.E.: The electrical resistivity log as an aid in determining some reservoir characteristics. Trans AIME. 146(01), 54–62 (1942). https://doi.org/10.2118/942054-G
Backhaus, S., Turitsyn, K., Ecke, R.E.: Convective instability and mass transport of diffusion layers in a Hele-Shaw geometry. Phys. Rev. Lett. 106(10), 104501 (2011). https://doi.org/10.1103/PhysRevLett.106.104501
Brouzet, C., Méheust, Y., Meunier, P.: CO2 convective dissolution in a three-dimensional granular porous medium: an experimental study. Phys. Rev. Fluids. 7(3), 033802 (2022). https://doi.org/10.1103/PhysRevFluids.7.033802
Bruggeman, D.G.: Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 416(7), 636–664 (1935). https://doi.org/10.1002/andp.19354160705
Cooper, C.A., Crews, J.B., Schumer, R., Breitmeyer, R.J., Voepel, H., Decker, D.L.: Experimental investigation of transient thermal convection in porous media. Transp. Porous Media 104(2), 335–347 (2014). https://doi.org/10.1007/s11242-014-0337-0
Croccolo, F., Brogioli, D.: Quantitative Fourier analysis of schlieren masks: the transition from shadowgraph to schlieren. Appl. Opt. 50(20), 3419–3427 (2011). https://doi.org/10.1364/AO.50.003419
Croccolo, F., Bataller, H., Scheffold, F.: A light scattering study of non equilibrium fluctuations in liquid mixtures to measure the Soret and mass diffusion coefficient. J. Chem. Phys. 137(23), 234202 (2012). https://doi.org/10.1063/1.4771872
Croccolo, F., Scheffold, F., Vailati, A.: Effect of a marginal inclination on pattern formation in a binary liquid mixture under thermal stress. Phys. Rev. Lett. 111(1), 014502 (2013). https://doi.org/10.1103/PhysRevLett.111.014502
Cushman-Roisin B, Mihailovic´ CG& DT. 2008. Environmental fluid mechanics: current issues and future outlook. In: Fluid Mechanics of Environmental Interfaces. Taylor & Francis
De N, Singh N, Fulcrand R, Méheust Y, Meunier P, Nadal F. 2022. Two-dimensional micromodels for studying the convective dissolution of carbon dioxide in 2D water-saturated porous media. 22:4645–4655. https://doi.org/10.1039/D2LC00540Aï.
De Paoli, M., Alipour, M., Soldati, A.: How non-Darcy effects influence scaling laws in Hele-Shaw convection experiments. J. Fluid Mech. (2020). https://doi.org/10.1017/jfm.2020.229
De Paoli M, Howland CJ, Verzicco R, Lohse D. 2023. Convective dissolution in confined porous media. J Fluid Mech.
Dentz, M., Borgne, T.L., Lester, D.R., Barros, F.P.J. de.: Mixing in groundwater. In: The Handbook of Groundwater Engineering. 3rd ed. CRC Press, Boca Raton (2016)
Dhar, J., Meunier, P., Nadal, F., Méheust, Y.: Convective dissolution of carbon dioxide in two- and three-dimensional porous media: the impact of hydrodynamic dispersion. Phys. Fluids 34(6), 064114 (2022). https://doi.org/10.1063/5.0086370
Dijksman, J.A., Rietz, F., Lorincz, K.A., Van Hecke, M., Losert, W.: Refractive index matched scanning of dense granular materials. Rev. Sci. Instrum. (2012). https://doi.org/10.1063/1.3674173
Dullien, F.A.L.: 3 - Pore Structure. In: Dullien, F.A.L. (ed.) Porous Media, pp. 75–155. Academic Press, Cambridge (1979)
Durucan, S., Shi, J.-Q., Sinayuc, C., Korre, A.: In Salah CO2 storage JIP: carbon dioxide plume extension around KB-502 well–new insights into reservoir behaviour at the In Salah storage site. Energy Procedia. 4, 3379–3385 (2011). https://doi.org/10.1016/j.egypro.2011.02.260
Eckel, A.-M., Liyanage, R., Kurotori, T., Pini, R.: Spatial moment analysis of convective mixing in three-dimensional porous media using X-ray CT images. Ind. Eng. Chem. Res. 62(1), 762–774 (2023). https://doi.org/10.1021/acs.iecr.2c03350
Emami Meybodi, H., Hassanzadeh, H.: Stability analysis of two-phase buoyancy-driven flow in the presence of a capillary transition zone. Phys. Rev. E – Stat. Nonlinear Soft Matter Phys. 87(3), 1–14 (2013). https://doi.org/10.1103/PhysRevE.87.033009
Ergun, S., Orning, A.A.: Fluid flow through randomly packed columns. Ind. Eng. Chem. Res. 41(6), 1179–1184 (1949)
Fruton, P., Nauruzbaeva, A., Bataller, H., Giraudet, C., Vailati, A., Croccolo, F.: Convective dissolution of carbon dioxide into brine in a three-dimensional free medium. Phys. Rev. Fluids. 8(2), 023503 (2023). https://doi.org/10.1103/PhysRevFluids.8.023503
Grathwohl P.: Measured diffusion coefficients. In: Grathwohl P (eds.) Diffusion in Natural Porous Media: Contaminant Transport, Sorption/Desorption and Dissolution Kinetics (Topics in Environmental Fluid Mechanics). Springer, Boston, pp. 83–127 (1998). https://doi.org/10.1007/978-1-4615-5683-1_4.
Haughey, D.P., Beveridge, G.S.G.: Structural properties of packed beds: a review. Can. J. Chem. Eng. 47(2), 130–140 (1969). https://doi.org/10.1002/cjce.5450470206
Hewitt, D.R.: Vigorous convection in porous media: vigorous convection in porous media. Proc. R. Soc. Math. Phys. Eng. Sci. (2020). https://doi.org/10.1098/rspa.2020.0111rspa20200111
Johnson, G.A., Shattuck, M.D., Behringer, R.P., Johnson, G.A., Georgiadis, J.G.: Convection and flow in porous media. Part 1. Visualization by magnetic resonance imaging. J. Fluid Mech. 332, 215–245 (1997)
Kaempf, J.: Advanced Ocean Modelling: Using Open-Source Software. Springer, Berlin (2010)
Liyanage, R., Cen, J., Krevor, S., Crawshaw, J.P., Pini, R.: Multidimensional observations of dissolution-driven convection in simple porous media using X-ray CT scanning. Transp. Porous Media 126(2), 355–378 (2019). https://doi.org/10.1007/s11242-018-1158-3
Liyanage, R., Russell, A., Crawshaw, J.P., Krevor, S.: Direct experimental observations of the impact of viscosity contrast on convective mixing in a three-dimensional porous medium. Phys. Fluids (2020). https://doi.org/10.1063/5.0006679
Messlinger, S., Schöpf, W., Rehberg, I.: Transient diffusive boundary layers at high Rayleigh numbers in simple and double diffusive fluids: latency time scaling for the convection onset. Int. J. Heat Mass Transf. 62, 336–349 (2013). https://doi.org/10.1016/j.ijheatmasstransfer.2013.02.076
Michel-Meyer, I., Shavit, U., Tsinober, A., Rosenzweig, R.: The role of water flow and dispersive fluxes in the dissolution of CO2 in deep saline aquifers. Water Resour. Res. 56(11), e2020WR028184 (2020). https://doi.org/10.1029/2020WR028184
Nakanishi, Y., Hyodo, A., Wang, L., Suekane, T.: Experimental study of 3D Rayleigh-Taylor convection between miscible fluids in a porous medium. Adv. Water Resour. 97, 224–232 (2016). https://doi.org/10.1016/j.advwatres.2016.09.015
Nield, D.A., Bejan, A.: Convection in Porous Media. Springer, New York (2013)
Ringrose, P.S., Mathieson, A.S., Wright, I.W., Selama, F., Hansen, O., Bissell, R., Saoula, N., Midgley, J.: The In Salah CO2 storage project: lessons learned and knowledge transfer. Energy Procedia. 37, 6226–6236 (2013). https://doi.org/10.1016/j.egypro.2013.06.551
Ringrose, P., Atbi, M., Mason, D., Espinassous, M., Myhrer, Ø., Iding, M., Mathieson, A., Wright, I.: Plume development around well KB-502 at the In Salah CO2 storage site. First Break (2009). https://doi.org/10.3997/1365-2397.27.1295.28744
Shattuck, M.D., Behringer, R.P., Johnson, G.A., Georgiadis, J.G.: Onset and stability of convection in porous media: visualization by magnetic resonance imaging. Phys. Rev. Lett. 75(10), 1934 (1995). https://doi.org/10.1103/PhysRevLett.75.1934
Teng, Y., Wang, P., Jiang, L., Liu, Y., Song, Y., Wei, Y.: An experimental study of density-driven convection of fluid pairs with viscosity contrast in porous media. Int. J. Heat Mass Transf. (2020). https://doi.org/10.1016/j.ijheatmasstransfer.2020.119514
Tsai, P.A., Riesing, K., Stone, H.A.: Density-driven convection enhanced by an inclined boundary: implications for geological CO2 storage. Phys. Rev. E – Stat. Nonlinear Soft. Matter Phys. 87(1), 1–4 (2013). https://doi.org/10.1103/PhysRevE.87.011003
Wang, L., Nakanishi, Y., Hyodo, A., Suekane, T.: Three-dimensional structure of natural convection in a porous medium: effect of dispersion on finger structure. Int. J. Greenh. Gas. Control. 53, 274–283 (2016). https://doi.org/10.1016/j.ijggc.2016.08.018
Wang, S., Cheng, Z., Zhang, Y., Jiang, L., Liu, Y., Song, Y.: Unstable density-driven convection of CO2 in homogeneous and heterogeneous porous media with implications for deep saline aquifers. Water Resour. Res. (2021). https://doi.org/10.1029/2020WR028132
Wu, Y.-S.: Multiphase fluids in porous media. In: Wu, Y.-S. (ed.) Multiphase Fluid Flow in Porous and Fractured Reservoirs, pp. 15–27. Gulf Professional Publishing, Boston (2016)
Wu, W., Klein, T., Kerscher, M., Rausch, M.H., Koller, T.M., Giraudet, C., Fröba, A.P.: Diffusivities in 1-alcohols containing dissolved H2, He, N2, CO, or CO2 Close to infinite dilution. J. Phys. Chem. B 123(41), 8777–8790 (2019). https://doi.org/10.1021/acs.jpcb.9b06211
Yin, P., Song, H., Ma, H., Yang, W., He, Z., Zhu, X.: The modification of the Kozeny–Carman equation through the lattice Boltzmann simulation and experimental verification. J. Hydrol. 609, 127738 (2022). https://doi.org/10.1016/j.jhydrol.2022.127738
Funding
This research was carried out under the framework of the E2S UPPA Hub Newpores and Industrial Chair CO2ES, supported by the Investissements d’Avenir French program managed by ANR (No. ANR161DEX0002). We also acknowledge support from the Petroleum Technology Development Fund (PTDF) Nigeria.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study, conception, and design. Material preparation, data collection, and analysis were performed by HI, PF, and CG. The first draft of the manuscript was written by HI, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Density of Fluid Mixtures
In the study of the thermodynamic mixing of toluene and 1-hexanol, (Almasi 2021) conducted density and viscosity measurements using a scanning voltage microscopy (SVM) 3000 Stabinger viscometer. For all the compositions and a temperature of 293.2 K, we plotted the density data as shown in Fig. 9a and fitted them through a density equation as follows:
The Bias \(=\frac{1}{N}\sum_{i=1}^{{N}_{c}}100\left(1-\frac{{\rho }_{calc}}{{\rho }_{exp}}\right)\), the mean absolute deviation \(AAD=\frac{1}{N}{\sum }_{i=1}^{{N}_{c}}100\left|1-\frac{{\rho }_{calc}}{{\rho }_{exp}}\right|\) and the maximum deviation \({\Delta }_{\max}=\max\left(100\left|1-\frac{{\rho }_{calc}}{{\rho }_{exp}}\right|\right)\) were estimated from a total of N = 12 experimental points and the results are reported in Table 5. The relative deviations of our calculations from the experimental measurements are shown in Fig. 9b.
Appendix B: Calculation of the Molecular Diffusion Coefficient
A correlation for the computation of the mass diffusion coefficient for binary mixtures of n-alkanes and systems involving gases dissolved in alcohols at infinite dilution was proposed by (Wu et al. 2019). The correlation was generated using more than 300 datasets from various light-scattering experiments and equilibrium molecular simulations. Below is the correlation for determining the molecular diffusion coefficient of a liquid mixture.
where \(\psi\) is the association factor, T is the temperature (K), \({M}_{1}\) and \({M}_{2}\) are the molar masses of the solute and solvent (g mol−1), respectively, \({v}_{2}\) is the kinematic viscosity of the pure solvent (m2 s−1), \({\omega }_{1}\) is the acentric factor of the solute, and \({\mu }_{2}\) is the total dipole moment of the solvent (Debye). When a fluid's thermodynamic state changes, the dipole moment of the molecule must also change; this information is frequently unavailable.
The uncertainty in using this correlation comes from the value of the dipole moment because it is assumed that the liquid structure is independent of the systems, and the association factor does not contain any information about the solute.
In this study, we used the acentric factor of toluene for the composition of the injected fluids (toluene/1-hexanol mixtures) during the diffusion coefficient calculation, because the wt% of toluene was higher than 80% in all cases. The total dipole for the mixtures was taken to be equal to 1, and the dynamic viscosity was derived from the fitting of the experimental data from Almasi (2021). The density data used to calculate the kinematic viscosity were also obtained from the fitting of experimental data from Almasi (2021). The average molecular weight was calculated using the formula \(\sum ({x}_{i}{M}_{i})/{n}_{i}\), where \({x}_{i}\)= wt% of i component; \({M}_{i}\) = molar mass of i component; \({n}_{i}\) = number of i component.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Imuetinyan, H., Fruton, P., Giraudet, C. et al. Convective Plume Spreading in Model Transparent Porous Media. Transp Porous Med (2024). https://doi.org/10.1007/s11242-024-02090-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11242-024-02090-z