Abstract
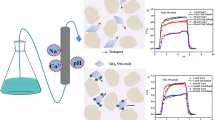
This study examines the influence of pH and ionic strength (\(I_{\mathrm{S}}\)) on the cotransport of graphene oxide (GO) nanoparticles and kaolinite (KGa-1b) colloids. Several flowthrough experiments were conducted in water-saturated columns, packed with either glass beads or quartz sand, in order to determine the transport behavior of GO and KGa-1b independently, as well as the cotransport behavior of GO together with KGa-1b. Various water chemistry conditions (\(\hbox {pH}=4, 7, 10\) and \(I_{\mathrm{S}}=7, 12, 27\,\hbox {mM}\)) were considered. Collision efficiencies were calculated using the classical colloid filtration theory. Interaction energy profiles between GO nanoparticles or KGa-1b colloids and glass beads or quartz sand were constructed for the various experimental conditions, by using measured zeta potentials and applying the classical Derjaguin–Landau–Verwey–Overbeek theory. The cotransport experimental breakthrough data suggested that by lowering the pH, the retention of GO nanoparticles is enhanced, due to a possible increase in heteroaggregation between GO nanoparticles and KGa-1b colloids. Also, by increasing the \(I_{\mathrm{S}}\) values, the retention of GO nanoparticles was slightly increased. The mass recovery of GO nanoparticles was reduced, and the transport of GO nanoparticles was retarded in the presence of KGa-1b colloids. Furthermore, the retention of GO nanoparticles was greater for columns packed with quartz sand than glass beads.








Similar content being viewed by others
Abbreviations
- \({A}_{123}\) :
-
Combined Hamaker constant, \(\hbox {M L}^{2}/\hbox {t}^{2}\)
- \({C}_{\mathrm{GO}}\) :
-
Concentration of GO nanoparticles, \(\hbox {M/L}^{3}\)
- \({C}_{0({\mathrm{GO}})}\) :
-
Initial concentration of GO nanoparticles, \(\hbox {M/L}^{3}\)
- \({C}_{{i}}\) :
-
Concentration of particles i, \(\hbox {M/L}^{3}\)
- \({C}_{{i}}^{*}\) :
-
Concentration of particles i attached onto the solid matrix, \(\mathrm{M}_{\mathrm{i}}/\mathrm{M}_{\mathrm{s}}\)
- \({C}_{0({{i}})}\) :
-
Initial concentration of particles i, \(\hbox {M/L}^{3}\)
- \({C}_{\mathrm{KGa{\text {-}}1b}}\) :
-
Concentration of KGa-1b colloids, \(\hbox {M/L}^{3}\)
- \({C}_{0({\mathrm{KGa{\text {-}}1b}})}\) :
-
Initial concentration of KGa-1b colloids, \(\hbox {M/L}^{3}\)
- \({d}_{\mathrm{c}}\) :
-
Collector diameter, L
- \({d}_{\mathrm{p}}\) :
-
Colloidal particle diameter, L
- e :
-
Elementary charge (Coulomb), C
- g :
-
Acceleration due to gravity, \(\hbox {L/t}^{2}\)
- h :
-
Separation distance between two approaching surfaces, L
- i :
-
Subscript indicating the GO nanoparticles or KGa-1b colloids
- \({I}_{\mathrm{S}}\) :
-
Ionic strength (mol/L)
- \({k}_{\mathrm{B}}\) :
-
Boltzmann’s constant, \(\hbox {M L}^{2}/(\hbox {t}^{2}\hbox { T})\)
- L :
-
Length of packed column, L
- \({M}_{{n}}\) :
-
nth normalized temporal moment, defined in Eq. (4), \(\mathrm{t}^{{n}}\)
- \(\mathrm{M}_{\mathrm{i}}\) :
-
Mass of particles i, \(\mathrm{M}_{\mathrm{i}}\)
- \(\mathrm{M}_{\mathrm{s}}\) :
-
Mass of the solid matrix, \(\mathrm{M}_{\mathrm{s}}\)
- \({M}_{\mathrm{r(i)}}\) :
-
Mass recovery in the outflow of particles i (%)
- \({M}_{\mathrm{r(tr)}}\) :
-
Tracer mass recovery in the outflow (%)
- n :
-
Subscript indicating the order of the moment (−)
- \({N}_{\mathrm{A}}\) :
-
Avogadro’s number (1/mol)
- \({r}_{{{i-i}}^{*}}\) :
-
Rate coefficient of particle attachment onto the solid matrix, 1/t
- \({r}_{{{i}}^{*}{{-i}}}\) :
-
Rate coefficient of particle detachment from the solid matrix, 1/t
- \({r}_{\mathrm{p}}\) :
-
Colloidal particle radius, L
- RB:
-
Ratio of \({M}_{\mathrm{r(i)}}\), relative to \({M}_{\mathrm{r(tr)}}\), (−)
- t :
-
Time, (t)
- \({t}_{\mathrm{p}}\) :
-
Time period of constant source concentration, t
- T :
-
Temperature in Kelvin, (T)
- U :
-
Pore water velocity, L/t
- x :
-
Cartesian coordinate, L
- \({\alpha }\) :
-
Collision efficiency (−)
- \(\varepsilon \) :
-
Dielectric constant of the suspending liquid [\(\hbox {C}^{2}/(\hbox {J}\,\hbox {m})\)]
- \(\varepsilon _{\mathrm{r} }\) :
-
Dimensionless relative dielectric constant of the suspending liquid (−)
- \(\varepsilon _{0 }\) :
-
Permittivity of free space [\(\hbox {C}^{2}/(\hbox {J}\,\hbox {m})\)]
- \(\eta _{0 }\) :
-
Dimensionless single-collector removal efficiency for favorable deposition (−)
- \(\theta \) :
-
Porosity (−)
- \(\kappa \) :
-
Debye–Huckel length, 1/L
- \(\mu _{\mathrm{w}}\) :
-
Absolute fluid viscosity, M/(Lt)
- \(\rho _{\mathrm{b}}\) :
-
Dry bulk density, \(\hbox {M/L}^{3}\)
- \(\rho _{\mathrm{f}}\) :
-
Fluid density, \(\hbox {M/L}^{3}\)
- \(\rho _{\mathrm{p}}\) :
-
Particle density, \(\hbox {M/L}^{3}\)
- \(\varPhi _{\mathrm{Born}}\) :
-
Born potential energy (J), \(\hbox {M L}^{2}/\hbox {t}^{2}\)
- \(\varPhi _{\mathrm{dl}}\) :
-
Double layer potential energy (J), \(\hbox {M L}^{2}/\hbox {t}^{2}\)
- \(\varPhi _{\mathrm{max1}}\) :
-
Primary maximum of the total interaction energy (J), \(\hbox {M L}^{2}\hbox {/t}^{2}\)
- \(\varPhi _{\mathrm{min1}}\) :
-
Primary minimum of total interaction energy (J), \(\hbox {M L}^{2}\hbox {/t}^{2}\)
- \(\varPhi _{\mathrm{min2}}\) :
-
Secondary minimum of total interaction energy (J), \(\hbox {M L}^{2}\hbox {/t}^{2}\)
- \(\varPhi _{\mathrm{vdW}}\) :
-
Van der Waals potential energy (J), \(\hbox {M L}^{2}\hbox {/t}^{2}\)
- \(\varPsi _{1}\) :
-
Stern potential of GO nanoparticles (V)
- \(\varPsi _{2}\) :
-
Stern potential of KGa-1b colloids (V)
References
Ackler, H.D., French, R.H., Chiang, Y.M.: Comparisons of Hamaker constants for ceramic systems with intervening vacuum or water: from force laws and physical properties. J. Colloid Interface Sci. 179, 460–469 (1996)
Bayat, A.E., Junin, R., Mohsin, R., Hokmabadi, M., Shamshirband, S.: Influence of clay particles on \(\text{ Al }_{2}\text{ O }_{3}\) and \(\text{ TiO }_{2}\) nanoparticles transport and retention through limestone porous media: measurements and mechanisms. J. Nanopart. Res. 17(219), 1–14 (2015). doi:10.1007/s11051-015-3031-4
Bradford, S.A., Bettahar, M., Simunek, J., Genuchten, M.T.: Straining and attachment of colloids in physically heterogeneous porous media. Vadose Zone J. 3, 384–394 (2004)
Cai, L., Tong, M., Wang, X., Kim, H.: Influence of clay particles on the transport and retention of titanium dioxide nanoparticles in quartz sand. Environ. Sci. Technol. 48(13), 7323–7332 (2014). doi:10.1021/es5019652
Cai, L., Zhu, J., Hou, Y., Tong, M., Kim, H.: Influence of gravity on transport and retention of representative engineered nanoparticles in quartz sand. J. Contam. Hydrol. 181, 153–160 (2015)
Chen, K.L., Elimelech, M.: Influence of humic acid on the aggregation kinetics of fullerene \((\text{ C }_{60})\) nanoparticles in monovalent and divalent electrolyte solutions. J. Colloid Interface Sci. 309(1), 126–134 (2007). doi:10.1016/j.jcis.2007.01.074
Chen, Y., Ren, C., Ouyang, S., Hu, X., Zhou, Q.: Mitigation in multiple effects of graphene oxide toxicity in Zebrafish embryogenesis driven by humic acid. Environ. Sci. Technol. 49(16), 10147–10154 (2015). doi:10.1021/acs.est.5b02220
Chrysikopoulos, C.V., Katzourakis, V.E.: Colloid particle size-dependent dispersivity. Water Resour. Res. 51, 4668–4683 (2015). doi:10.1002/2014WR016094
Chrysikopoulos, C.V., Syngouna, V.I.: Attachment of bacteriophages MS2 and \(\Phi \) X174 onto kaolinite and montmorillonite: extended-DLVO interactions. Colloids Surf. B 92, 74–83 (2012). doi:10.1016/j.colsurfb.2011.11.028
Chrysikopoulos, C.V., Syngouna, V.I.: Effect of gravity on colloid transport through water-saturated columns packed with glass beads: modeling and experiments. Environ. Sci. Technol. 48, 6805–6813 (2014). doi:10.1021/es501295n
Chrysikopoulos, C.V., Roberts, P.V., Kitanidis, P.K.: One-dimensional solute transport in porous media with partial well-to-well recirculation: application to field experiments. Water Resour. Res. 26(6), 1189–1195 (1990)
Choi, N.H., Kim, D.J., Kim, S.B.: Quantification of bacterial mass recovery as a function of pore-water velocity and ionic strength. Res. Microbiol. 158, 70–78 (2007)
Cornelis, G., Pang, L., Doolette, C., Kirby, J.K., McLaughlin, M.J.: Transport of silver nanoparticles in saturated columns of natural soils. Sci. Total Environ. 463–464, 120–130 (2013). doi:10.1016/j.scitotenv.2013.05.089
Derjaguin, B.V., Landau, L.: Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solution of electrolytes. Acta Physicochim. URSS 14, 633–662 (1941)
Dreyer, D.R., Park, S., Bielawski, C.W., Ruoff, R.S.: The chemistry of graphene oxide. Chem. Soc. Rev. 39, 228–240 (2010). doi:10.1039/b917103g
Elimelech, M., Gregory, J., Jia, X., Williams, R.A.: Particle Deposition & Aggregation: Measurement Modelling and Simulation. Butterworth-Heinemann Ltd, Oxford (1995)
Fan, W., Jiang, X.H., Yang, W., Geng, Z., Huo, M.X., Liu, Z.M., Zhou, H.: Transport of graphene oxide in saturated porous media: effect of cation composition in mixed Na-Ca electrolyte systems. Sci. Total Environ. 511, 509–515 (2015a). doi:10.1016/j.scitotenv.2014.12.099
Fan, W., Jiang, X.H., Lu, Y., Huo, M., Lin, S., Geng, Z.: Effects of surfactants on graphene oxide nanoparticles transport in saturated porous media. J. Environ. Sci. 35, 12–19 (2015b). doi:10.1016/j.jes.2015.02.007
Feriancikova, L., Xu, S.: Deposition and remobilization of oxide within saturated sand packs. J. Hazard. Mater. 235–236, 194–200 (2012)
Han, Z., Zhang, F., Lin, D., Xing, B.: Minerals affect the stability of surfactant-facilitated carbon nanotube suspensions. Environ. Sci. Technol. 42, 6869–6875 (2008)
Haynes, W.M. (ed.): CRC Handbook of Chemistry and Physics, 94th edn. CRC Press, Boca Raton (2013)
He, C., Shi, Z.Q., Ma, L., Cheng, C., Nie, C.X., Zhoua, M., Zhao, C.S.: Graphene oxide based heparin-mimicking and hemocompatible polymeric hydrogels for versatile biomedical applications. J. Mater. Chem. B 3, 592–602 (2015). doi:10.1039/C4TB01806K
Ho, Y.-S.: Review of second-order models for adsorption systems. J. Hazard. Mater. 136(3), 681–689 (2006)
Hegab, H.M., Zou, L.: Graphene oxide-assisted membranes: fabrication and potential applications in desalination and water purification. J. Membr. Sci. 484, 95–106 (2015). doi:10.1016/j.memsci.2015.03.011
Hendry, M.J., Lawrence, J.R., Maloszewski, P.: Effect of velocity on the transport of two bacteria through saturated sand. Ground Water 37, 103–112 (1999)
Hu, C., Wang, Q., Zhao, H., Wang, L., Guo, S., Li, X.: Ecotoxicological effects of graphene oxide on protozoan Euglena gracilis. Chemosphere 128, 184–190 (2015). doi:10.1016/j.chemosphere.2015.01.040
Jiang, X., Wang, X., Tong, M., Kim, H.: Initial transport and retention behaviors of ZnO nanoparticles in quartz sand porous media coated with Escherichia coli biofilm. Environ. Pollut. 174, 38–49 (2013)
Jian-Zhou, H., Cheng-Cheng, L., Deng-Jun, W., Zhou, D.M.: Biofilms and extracellular polymeric substances mediate the transport of graphene oxide nanoparticles in saturated porous media. J. Hazard. Mater. 300, 467–474 (2015). doi:10.1016/j.jhazmat.2015.07.026
Katzourakis, V.E., Chrysikopoulos, C.V.: Mathematical modeling of colloid and virus cotransport in porous media. Adv. Water Resour. 68, 62–73 (2014). doi:10.1016/j.advwatres.2014.03.001
Katzourakis, V.E., Chrysikopoulos, C.V.: Modeling dense-colloid and virus cotransport in three-dimensional porous media. J. Contam. Hydrol. 181, 102–113 (2015). doi:10.1016/j.jconhyd.2015.05.010
Katzourakis, V.E., Chrysikopoulos, C.V.: Fitting the transport and attachment of dense biocolloids in one-dimensional porous media: ColloidFit. Groundwater (2017). doi:10.1111/gwat.12501
Keller, A., McFerran, S., Lazareva, A., Suh, S.: Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 15(6), 1–17 (2013). doi:10.1007/s11051-013-1692-4
Kim, H.J., Phenrat, T., Tilton, R.D., Lowry, G.V.: Effect of kaolinite, silica fines and pH on transport of polymer-modified zero valent iron nano-particles in heterogeneous porous media. J. Colloid Interface Sci. 370, 1–10 (2012a). doi:10.1016/j.jcis.2011.12.059
Kim, J., Cote, L.J., Huang, J.: Two dimensional soft material: new faces of graphene oxide. Acc. Chem. Res. 45(8), 1356–1364 (2012b). doi:10.1021/ar300047s
Kim, C., Lee, S.: Effect of seepage velocity on the attachment efficiency of \(\text{ TiO }_{2}\) nanoparticles in porous media. J. Hazard. Mater. 279, 163–168 (2014)
Lanphere, J.D., Luth, C.J., Walker, S.L.: Effects of solution chemistry on the transport of graphene oxide in saturated porous media. Environ. Sci. Technol. 47, 4255–4261 (2013)
Lanphere, J.D., Rogers, B., Luth, C.J., Bolster, C.H., Walker, S.L.: Stability and transport of graphene oxide nanoparticles in groundwater and surface water. Environ. Eng. Sci. 31(7), 350–359 (2014). doi:10.1089/ees.2013.0392
Liang, S., Xu, S., Zhang, D., He, J., Chu, M.: Reproductive toxicity of nanoscale graphene oxide in male mice. Nanotoxicology 9(1), 92–105 (2015). doi:10.3109/17435390.2014.893380
Liu, H.H., Cohen, Y.: Multimedia environmental distribution of engineered nanomaterials. Environ. Sci. Technol. 48, 3281–3292 (2014). doi:10.1021/es405132z
Liu, L., Gao, B., Wu, L., Morales, V.L., Yang, L., Zhou, Z., Wang, H.: Deposition and transport of graphene oxide in saturated and unsaturated porous media. Chem. Eng. J. 229, 444–449 (2013)
Liu, L., Gao, B., Wu, L., Sun, Y., Zhou, Z.: Effects of surfactant type and concentration on graphene retention and transport in saturated porous media. Chem. Eng. J. 262, 1187–1191 (2015). doi:10.1016/j.cej.2014.10.032
Loveland, J.P., Ryan, J.N., Amy, G.L., Harvey, R.W.: The reversibility of virus attachment to mineral surfaces. Colloids Surf. A 107, 205–221 (1996)
Lv, X., Gao, B., Sun, Y., Shi, X., Xu, H., Wu, J.: Effects of humic acid and solution chemistry on the retention and transport of cerium dioxide nanoparticles in saturated porous media. Water Air Soil Pollut. 225, 2167 (2014). doi:10.1007/s11270-014-2167-7
Lyklema, J.: Fundamentals of Interface and Colloid Science. Academic Press, London (1991)
Mitropoulou, P.N., Syngouna, V.I., Chrysikopoulos, C.V.: Transport of colloids in unsaturated packed columns: role of ionic strength and sand grain size. Chem. Eng. J. 232, 237–248 (2013)
Pruett, R.J., Webb, H.L.: Sampling and analysis of KGa-1b well-crystallized kaolin source clay. Clays. Clay. Miner. 41(4), 514–519 (1993)
Rajagopalan, R., Tien, C.: Trajectory analysis of deep-bed filtration with the sphere-in-cell porous media model. AIChE J. 22, 523–533 (1976)
Reddy, D.A., Ma, R., Choi, M.Y., Kim, T.K.: Reduced graphene oxide wrapped ZnS–Ag2S ternary composites synthesized via hydrothermal method: Applications in photocatalyst degradation of organic pollutants. Appl. Surf. Sci. 324, 725–735 (2015). doi:10.1016/j.apsusc.2014.11.026
Rong, X., Huanga, Q., He, X., Chen, H., Cai, P., Liang, W.: Interaction of Pseudomonas putida with kaolinite and montmorillonite: a combination study byequilibrium adsorption, ITC, SEM and FTIR. Colloids Surf. B 64, 49–55 (2008)
Seabra, A.B., Paula, A.J., de Lima, R., Alves, O.L., Durán, N.: Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 27(2), 159–168 (2014). doi:10.1021/tx400385x
Sim, Y., Chrysikopoulos, C.V.: Analytical models for one-dimensional virus transport in saturated porous media. Water Resour. Res. 31(5), 1429–1437 (1995). doi:10.1029/95WR00199
Sim, Y., Chrysikopoulos, C.V.: Three-dimensional analytical models for virus transport in saturated porous media. Transp. Porous Med. 30, 87–112 (1998). doi:10.1023/A:1006596412177
Song, Z., Wang, X., Zhu, G., Nian, Q., Zhou, H., Yang, D., Qin, C., Tang, R.: Virus capture and destruction by label-free graphene oxide for detection and disinfection applications. Small 11(9–10), 1171–1176 (2015). doi:10.1002/smll.201401706
Sotirelis, N.P., Chrysikopoulos, C.V.: Interaction between graphene oxide nanoparticles and quartz sand. Environ. Sci. Technol. 94(22), 13413–13421 (2015). doi:10.1021/acs.est.5b03496
Sotirelis, N.P., Chrysikopoulos, C.V.: Heteroaggregation of graphene oxide nanoparticles and kaolinite colloids. Sci. Total Environ. 579, 736–744 (2017). doi:10.1016/j.scitotenv.2016.11.034
Stankovich, S., Dikin, D.A., Dommett, G.H., Kohlhaas, K.M., Zimney, E.J., Stach, E.A., Piner, R.D., Nguyen, S.T., Ruoff, R.S.: Graphene-based composite materials. Nature 442, 282–285 (2006). doi:10.1038/nature04969
Stephan, E.A., Chase, G.G.: A preliminary examination of zeta potential and deep bed filtration activity. Sep. Purif. Technol. 21, 219–226 (2001)
Sun, Y., Gao, B., Bradford, S.A., Wu, L., Chen, H.: Transport, retention, and size perturbation of graphene oxide in saturated porous media: effects of input concentration and grain size. Water Res. 68, 24–33 (2015). doi:10.1016/j.watres.2014.09.025
Syngouna, V.I., Chrysikopoulos, C.V.: Cotransport of clay colloids and viruses in water saturated porous media. Colloids Surf. A. 416, 56–65 (2013). doi:10.1016/j.colsurfa.2012.10.018
Syngouna, V.I., Chrysikopoulos, C.V.: Cotransport of clay colloids and viruses through water-saturated vertically oriented columns packed with glass beads: gravity effects. Sci. Total Environ. 545–546, 210–218 (2016). doi:10.1016/j.scitotenv.2015.12.091
Tang, H., Zhang, J., Zhang, Y.J., Xiong, Q.Q., Tong, Y.Y., Li, Y., Wang, X.L., Gu, C.D., Tu, J.P.: Porous reduced graphene oxide sheet wrapped silicon composite fabricated by steam etching for lithium-ion battery application. J. Power Sources 286, 431–437 (2015). doi:10.1016/j.jpowsour.2015.03.185
Toda, K., Furue, R., Hayami, S.: Recent progress in applications of graphene oxide for gas sensing: a review. Anal. Chim. Acta. 878, 43–53 (2015). doi:10.1016/j.aca.2015.02.002
Tufenkji, N., Elimelech, M.: Correlation equation for predicting single-collector efficiency in physicochemical filtration in saturated porous media. Environ. Sci. Technol. 38(2), 529–536 (2014). doi:10.1021/es034049r
van Olphen, H., Fripiat, J.J.: Data Handbook for Clay Minerals and Other Non-metallic Minerals. Pergamon Press, Oxford (1979)
Vasiliadou, I.A., Chrysikopoulos, C.V.: Cotransport of Pseudomonas putida and kaolinite particles through water saturated columns packed with glass beads. Water Resour. Res. 47(2), W02543 (2011). doi:10.1029/2010WR009560
Verwey, E.J.W., Overbeek, J.T.G.: Theory of the Stability of Lyophobic Colloids, p. 205. Elsevier, Amsterdam (1948)
Voorn, D.J., Ming, W., Laven, J., Meuldijk, J., de With, G., van Herk, A.M.: Plate-sphere hybrid dispersions: heterocoagulation kinetics and DLVO evaluation. Colloids Surf. A. 294(1–3), 236–246 (2007). doi:10.1016/j.colsurfa.2006.08.022
Wang, H., Dong, Y.N., Zhu, M., Li, X., Keller, A.A., Wang, T., Li, F.: Heteroaggregation of engineered nanoparticles and kaolin clays in aqueous environments. Water Res. 80, 130–138 (2015). doi:10.1016/j.watres.2015.05.023
Wilson, M.J., Wilson, L., Patey, I.: The influence of individual clay minerals on formation damage of reservoir sandstones: a critical review with some new insights. Clay Miner. 49(2), 147–164 (2014). doi:10.1180/claymin.2014.049.2.02
Wu, L., Liu, L., Gao, B., Muñoz-Carpena, R., Zhang, M., Chen, H., Zhou, Z., Wang, H.: Aggregation kinetics of graphene oxides in aqueous solutions: experiments, mechanisms, and modeling. Langmuir 29, 15174–15181 (2013)
Wu, W., Yan, L., Wu, Q., Li, Y., Li, Q., Chen, S., Yang, Y., Gu, Z., Xu, H., Yin, Z.Q.: Evaluation of the toxicity of graphene oxide exposure to the eye. Nanotoxicology 10(9), 1329–1340 (2016). doi:10.1080/17435390.2016.1210692
Xiao, Y., Wiesner, M.R.: Transport and retention of selected engineered nanoparticles by porous media in the presence of a biofilm. Environ. Sci. Technol. 47(5), 2246–2253 (2013). doi:10.1021/es304501n
Yao, K.M., Habibian, M.T., O’Melia, C.R.: Water and waste water filtration. Concepts and Applications. Environ. Sci. Technol. 5(11), 1105–1112 (1971). doi:10.1021/es60058a005
Yoon, R.-H., Flin, D.H., Rabinovich, Y.I.: Hydrophobic interactions between dissimilar surfaces. J. Colloid Interface Sci. 185, 363–370 (1997)
Zhao, J., Liu, F., Wang, Z., Cao, X., Xing, B.: Heteroaggregation of graphene oxide with minerals in aqueous phase. Environ. Sci. Technol. 49(5), 2849–2857 (2015). doi:10.1021/es505605w
Zhou, D.D., Jiang, X.H., Lub, Y., Fan, W., Huo, M.X., Crittenden, J.C.: Cotransport of graphene oxide and Cu(II) through saturated porous media. Sci. Total Environ. 550, 717–726 (2016). doi:10.1016/j.scitotenv.2016.01.141
Acknowledgements
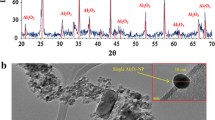
The authors are thankful for the various suggestions and thoughtful comments provided by V.E. Katzourakis. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. NGK-K conducted the energy-dispersive X-ray fluorescence analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chrysikopoulos, C.V., Sotirelis, N.P. & Kallithrakas-Kontos, N.G. Cotransport of Graphene Oxide Nanoparticles and Kaolinite Colloids in Porous Media. Transp Porous Med 119, 181–204 (2017). https://doi.org/10.1007/s11242-017-0879-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-017-0879-z