Abstract
In this study, response surface methodology (RSM) was used to optimize in vitro regeneration of the Brazilian micro sword (Lilaeopsis brasiliensis) aquatic plant, followed by data prediction and validation using machine learning algorithms. The basal salt, sucrose and Benzyaminopurine (BAP) concentrations were derived from Box-Behnken design of RSM. The response surface regression analysis revealed that 1.0 g/L MS + 0.1 mg/L BAP + 25 g/L sucrose was optimized for maximum regeneration (100%), shoot counts (63.2), and fresh weight (1.382 g). The RSM-based predicted scores were fairly similar to the actual scores, which were 100% regeneration, 63.39 shoot counts, and 1.44 g fresh weight. Pareto charts analysis illustrated the significance of MS for regeneration and fresh weight but remained insignificant. Conversely, MS × BAP was found to be the most crucial factor for the shoot counts, with MS coming in second and having a major influence. The analysis of the normal plot ascertained the negative impact of elevated MS concentration on shoot counts and enhanced shoot counts from the combination of MS × BAP. Results were further optimized by constructing contour and surface plots. The response optimizer tool demonstrated that maximum shoot counts of 63.26 and 1.454 g fresh weight can be taken from the combination of 1.0 g/L MS + 0.114 mg/L BAP + 23.94 g/L. Using three distinct performance criterias, the results of machine learning models showed that the multilayer perceptron (MLP) model performed better than the random forest (RF) model. Our findings suggest that the results may be utilized to optimize various input variables using RSM and verified via ML models.
Key message
-
Optimization of in vitro whole plant regeneration of Brazilian sword wood using response surface methodology
-
Data analysis through ANOVA, response surface regression anlaysis and machine learning
-
Graphical presentation of data via Pareto charts, normal plots, contour plots and surface plots for optimization
-
Better performance of ANN-based MLP model compared to decision tree based RF model
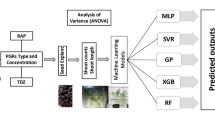
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquatic plants or hydrophytes live in water bodies (Saini et al. 2010) for their growth and reproduction. They are considered a crucial component of an aquatic environment because they supply oxygen, organic materials, food, and shelter to many living organisms (Oyedeji and Abowei 2012). Aquatic plants can also be used as ornamental aquarium and medicinal plants (Aasim et al. 2019), phytoremediation (Fletcher et al. 2020), waste water treatment (Ali et al. 2022), and water ecosystem biomonitoring (Zurayk et al. 2001). The demand for aquatic plants is growing rapidly, necessitating the development of an alternate method of mass propagation (Karatas et al. 2015). The mass production of aquatic plants is being carried out by the use of plant tissue culture techniques (Dogan 2022). However, the initial goal of enterprises involved in aquatic plants through plant tissue culture is to optimize plant tissue culture protocols for low-cost production. Brazilian micro sword (Lilaeopsis brasiliensis) is a creeping herb belonging to the Apiaceae family. It is one of the four species of Lilaeopsis genus that are recognized as an aquarium plant, and marketed with different names like water-umbel, water chives, and micro sword (Bone et al. 2011). The plant continues to multiply vertically through its rhizomes that are 0.2 to 1.0 mm in diameter and generate leaves from its node. The leaves are generally 1.0–7.0 cm long with hollow and elliptical structures. It is recognized as an appealing carpet plant in aquariums because of its distinctive leaf form (Ing et al. 2019).
Plant tissue culture relies on the optimization of input factors to produce an efficient, reliable, and cost-effective technique. The majority of the conventional techniques for optimizing inputs rely on analysis of variance. These traditional approaches frequently fail to offer precise optimization of input variables due to their inherent limitations. These issues can be elucidated by utilizing sophisticated statistical programs such as response surface methodology (RSM) or artificial intelligence (AI) based modeling. RSM often uses mathematical and statistical tools to optimize and validate operations (Anderson and Whitcomb 2016). The surface placement of data allows us to distribute them into different areas such as maximum, local, minimum, and ridge lines. In this way, two or more variables are used to find out the desired experimental responses or outcomes (Farooq et al. 2013; Wantala et al. 2012). Box-Behnken designs (BBD) and central composite designs (CCD) are two major experimental designs used for analysis (Koç and Kaymak-Ertekin 2010). In RSM, independent input variables (A, B, C….), interactions (AB, AC, and BC), and square of independent variables (A2, B2, and C2) are respectively expressed as the linear, interactive term coefficient, and quadratic term coefficient. The application of RSM allows certain advantages of data extraction with precise results in less time and relatively low experimental size. Whereas placement of data at the second level in the polynomial model may lead to certain disadvantages (Aydar 2018). The use of RSM in plant tissue culture is very limited irrespective of its significance.
Optimization of input factors for a given output parameter is important for in vitro regeneration protocol. However, the use of traditional statistical tools is not efficient in optimizing the data (Pepe et al. 2021) and demands new technologies for data prediction, validation, or optimization possibly due to noisy, and complicated datasets involving multifactorial processes. The advancement in the field of data science in the form of artificial intelligence or machine learning algorithms (Aasim et al. 2023b; Géron 2022) enables researchers to employ these models for complex data sets (Jafari et al. 2022; Sadat-Hosseini et al. 2022) involving multiple biological complex studies like in vitro regeneration (Aasim et al. 2023a) In plant tissue culture, optimizing the basal media and its contents is regarded as a critical procedure (Hesami and Jones 2021; Viswanathan et al. 2022) and regulated the organogenesis or embryogenesis (Hesami et al. 2020; Jamshidi et al. 2019). The simultaneous optimization of multiple input factors is extremely challenging due to the large experimental size, interactions, expense, and handling. This challenge can be handled by applying recent statistical approaches used for optimizing numerous input components with a small number of experimental treatments (Akin et al. 2017; Poothong and Reed 2014). The attained results or outcomes from the experiment can be predicted, validated or further optimized by employing artificial neural networks (ANN) or machine learning (ML) models. Keeping this in mind, this study was designed to optimize basal salt, sucrose, and hormonal concentrations for Brazilian sword wood plant, followed by examining the data using Artificial intelligence (AI) – based models. The ANN-based multilayer perceptron (MLP) and decision tree-based random forest (RF) models were employed on the data set generated through RSM. The performance of the models was analyzed by employing three different performance metrics, namely, coefficient of determination (R2), root mean squared error (RMSE), and mean absolute error (MAE).
Material and methods
Experimental setup
The explants were taken from in vitro propagated stock plants available in the plant tissue culture lab of Sivas University of Science and Technology, Sivas, Turkiye. The basal medium used in this study was comprised of different concentrations of Murashige and Skoog (MS, Duchefa) basal salts (Murashige and Skoog, 1962) with vitamins, Benzylaminopurine (BAP- Merck), and sucrose (Duchefa). The concentrations of all three were designed according to the design of experiment (DOE) using Box-Behnken (BB) model for RSM (Table 1). The variables were adjusted as 1.0–4.4 g/L MS, 0.1–1.5 mg/L BAP, and 10–40 g/L sucrose for DOE, and a total of 15 distinct combinations were opted for experiment. The culture medium was prepared according to the standard procedure by using distilled water. After adding sucrose and BAP, the pH of the medium was automated at 5.8 followed by adding agar (6.5 g/L). The mediums were autoclaved at 121 °C for 15 min and 1.5 atm. The 4–5 mm long node explants containing meristematic regions were taken under aseptic conditions and transferred to the glass jars (330 cc) containing growth medium. The nodal explants (Fig. 1a) were incubated in the growth room equipped with white light-emitting diodes (LEDs) with light intensity of (2030 LUX). The light was adjusted at 16-h photoperiod and growth chamber was automated at 24 ± 1 °C and 60.0% R:H. Five explants per replication and a total of 4 replications were cultured for 6 weeks before data on regeneration frequency, shoot counts per explant, and fresh weight per explant were collected. For shoot counts, the plant was separated manually containing its respective meristem at the basal end. The fresh weight was calculated by weighing all explants and dividing them by the number of explants in the replication. The data was analyzed via one-way ANOVA for individual factors (MS, BAP, sucrose) and multiple factors (MS × BAP × sucrose), and response surface design analysis. The data was further evaluated by creating various graphical charts and plots to study the influence of the two factors (MS × BAP; MS × sucrose; BAP × Sucrose). To determine the most important elements of the investigation, Pareto charts, normal plots, contour and surface plots, and response optimization were created. For statistical analysis and graphical display, two separate statistical programs were utilized. Minitab was used to create the Pareto charts, normal plots, and response optimization, while Design Expert was used to generate the contour and surface plots.
Machine learning analysis
The data obtained in this study was validated and forecasted using artificial intelligence-based models. The decision tree-based Random Forest (RF) model and the artificial neural network (ANN)–based multilayer perceptron (MLP) model were employed for data prediction and validation. The MLP is made up of many feedforward neural nodes connected in a feedforward fashion. The input, output, and fully integrated hidden level(s) can be one or multiple. Equation 1 presents the backpropagation, which is used to update the error-related biases and weights following training. (Katırcı et al. 2021).
y = observed value of data point k, K = number of samples.
The primary reasons why decision tree-based algorithms are used is because of their rapid analysis and high accuracy while being simple to use. Compared to linear models, it also enables significantly more accurate mapping of intricate non-linear interactions. The RF (Aggarwal 2018) is the most popular advance decision tree model in data science. It trains several trees simultaneously using bagging, or bootstrap aggregation. The outcome is influenced by nearly all trained trees, and the fundamental principle of the entire system is shown in Eq. 2 (Pavlov 2019).
y = data point value; n = sampling size (number).
The leave-one-out cross-validation (LOO-CV) method (Webb et al. 2011) was used to divide the data into two sections to evaluate the effectiveness of the employed algorithms. A particular kind of cross-validation known as LOO-CV occurs when the dataset being used has the same number of instances as folds. Every instance undergoes a unique learning process; testing and training are carried out on the chosen instance and the remaining instances, respectively. Because LOO-CV employs more training samples with each iteration, allowing the model to understand the data more fully, and performs better with small sample sizes. Finding the ideal hyperparameters through a grid search led to the establishment of the best model. The open-source code was coded using the Sklearn package (Pedregosa et al. 2011) and Python programming language (Van Rossum and Drake 2009) available as open-source. Three different performance measures, listed in Eqs. 3–5 were used to assess the performance of the two models.
where
\({Y}_{i}\) = measured value.
\({\widehat{Y}}_{i}\) = predicted value.
\(\overline{Y}\) = measured values mean.
n = count of samples.
R2 = Coefficient of determination; RMSE = Root mean square error; MAE = Mean Absolute Error.
Data preparation
Data scaling is a method used in the data preparation stage to bring various variables to a comparable scale to prevent certain traits from dominating others based only on their magnitudes. Converting the inputs into dimensionless or comparable distributions improves algorithm performance, data quality, and processing time. Using Eq. 6, all inputs were standardized to a unit standard deviation and zero mean before training and testing the models.
\({X}_{s}\): standardized value; Xi: actual data; μ: mean of the feature values; σ: standard deviation of the featured values.
Results
One-way ANOVA analysis
One-way analysis of individual input variables exhibited the insignificant impact of BAP and sucrose concentration on regeneration, shoot counts per explant and shoot length. The MS concentration exhibited a significant impact on shoot counts per explant (p0.005) and fresh weight explant (p0.005), with an insignificant impact on regeneration frequency (%). The MS concentration resulted in regeneration frequency of 95.00–100%, shoot counts per explant of 27.747–45.88, and fresh weight range of 0.589–1.470 g/explant (Table 1; Fig. 1a, b). Both shoot counts and fresh weight unveiled a similar pattern and decreased with an increase in MS concentration. The maximum shoot counts (45.88) and fresh weight (1.470 g) were recorded from a medium enriched with 1.0 mg/L MS. The BAP and sucrose concentration produced the maximum shoot counts and fresh weight at a low concentration of 0.1 mg/L BAP and 10.0 mg/L sucrose concentration. In vitro regenerated shoot clusters were successfully acclimatized (approximately 90.0%) in the aquariums and fresh water (Fig. 1c, d).
Response surface regression analysis
Response surface regression analysis was performed to investigate the impact of individual and multiple factors on in vitro regeneration. All p-values for regeneration (%) and fresh weight were recorded as insignificant with minimum p-values for both output parameters recorded for MS. The MS concentration (p0.018) and MS × BAP (p0.009) exerted a significant impact on the shoot counts. Whereas individual input (S, BAP) and interactions (MS × S, and S × BAP) exerted insignificant impact on shoot counts per explant (Table 2). Investigation of R2 (act) scores of output variables presented the order of shoot counts (R2-act: 89.35), regeneration frequency (R2-act: 61.81), and fresh weight (R2-act: 47.76). The regression equations of all output parameters are given below.
The MS (A), BAP (B), and sucrose (C) concentrations regulated the in vitro regeneration of L. brasiliensis. Different combinations of input variables resulted in variable morphological appearance (color and size) of in vitro regenerated shoots. However, results analyzed through RSM displayed fairly different responses to input factors, and the analysis of variance computed is presented in Table 3. Analysis of the model revealed the insignificant impact of input variables on all output parameters. A clear relationship between input and output parameters was observed irrespective of insignificant results. The data were analyzed by computing actual and predicted values (Table 3). In general, the differences between actual and predicted values of shoot counts per explant were very low compared to regeneration, and fresh weight. Results discerned that run 1, 4, 7, and 15 yielded predicted values of 99.25% regeneration. Considering shoot counts, the predicted scores were computed as 63.39 compared to 63.2 from run 8. Whereas, the predicted scores of 0.829 g were computed from run 13 compared to the actual score of 0.91. Overall comparison of actual and predicted values revealed the best results from Run 8 augmented with 1.0 g/L MS, 0.1 mg/L BAP, and 25.0 mg/L sucrose (Table 3).
Pareto and normal plot analysis
The results attained were also analyzed by constructing Pareto charts and normal plots to investigate the input variable with a superior impact on in vitro regeneration. Investigation of Pareto charts revealed a significance value of 2.571 and showed the variable placement of input variables. Results of Pareto charts revealed the order of significance as A > AB > AC > C > B > CC > BB > AA > BC (Fig. 2A) for regeneration (%), AB > A > AA > BC > C > AC > CC > B for shoot counts per explant (Fig. 2C), and A > C > AA > B > AB > AC > BC > BB > CC for fresh weight (Fig. 2E). Investigation of contour plots followed the order of the Pareto chart and distributed the data from top to bottom accordingly on the left to right of the line. The placement of input factors expressed the correlation between input variables on their respective output parameter. The input parameters on the right side illustrate the direct correlation between input and output parameters. Whereas inputs placed on the left side of the line reflect the inverse proportional relationship between input and output parameters. The input parameters expressed with red squares and blue circles respectively illustrate the significant and insignificant impact of input variables on their respective output parameter. Investigating regeneration (%) revealed the inverse proportional impact of A (MS) and BC (BAP × Sucrose) (Fig. 2B). Normal plot analysis of shoot counts (Fig. 2D) revealed the direct correlation of AB (MS × BAP-significant) and BC (BAP × Sucrose-insignificant). Whereas, input factors like B (BAP), C (Sucrose), and AC (MS × Sucrose) were insignificant and placed on the left side of the line. The A (MS-significant) factor was placed at the bottom but on the left side of the line. Results regarding fresh weight revealed the distribution of A, C, and B on the left side while AB, AC, and BC on the right side, but very close to the center line (Fig. 2F). It is concluded from Pareto charts and normal plots that MS is the most significant factor that affected the whole in vitro regeneration process.
Surface plots analysis
Optimization of two independent (input) variables on dependent (output) parameters was illustrated by constructing 3D surface plots (Fig. 3A-I). The surface plots optimized the input variables and distributed the data on the x-axis and y-axis, expressed with different colors. Results revealed that maximum regeneration (100%) can be obtained from 1.0–1.586 g/L MS and 0.1–1.022 mg/L BAP (Fig. 3A), and 1.0–1.586 g/L MS with 10–31.050 g/L sucrose (Fig. 3B). Whereas 1.22–1.50 g/L BAP and sucrose above 30 g/L may lead to 100% regeneration (Fig. 3C). Results of shoot counts revealed the variable number of shoot counts from input factors. The possible combination of 1.0–1.12 mg/L MS with 0.10–0.18 mg/L BAP may lead to more than 60 shoots (Fig. 3D) and 1.0–1.29 g/L MS with 10–40 g/L sucrose for more than 40 shoot counts (Fig. 3E). Whereas 0.1–0.33 mg/L BAP and 10.0–16.0 g/L sucrose combination may generate more than 35 shoots (Fig. 3F). Investigation on optimizing fresh weight illustrated the maximum of approximately 1.40 g fresh weight per explant from 1.0–1.08 g/L MS and 0.1–0.9 mg/L BAP combination (Fig. 3G). Whereas a combination of 2.05 MS and 10 g/L can produce a maximum fresh weight of 1.70 g (Fig. 3H). The combination of 0.83–1.49 mg/L BAP and 10.2–19.5 g/L sucrose led to a maximum fresh weight of 1.0 g per explant (Fig. 3I). The results of contour plots are presented as supplementary material (Figure S1).
Surface plots analysis of different input factors for in vitro regeneration Brazilian sword wood plants (a) MS × BAP (b) MS × sucrose (c) BAP × sucrose for regeneration, (d) MS × BAP (e) MS × sucrose (f) BAP × sucrose for shoot counts per explant, (g) MS × BAP (h) MS × sucrose, and (i) BAP × sucrose for fresh weight of Brazilian sword wood plants
Response surface optimization
Response Optimizer was used to find out the best possible inputs by setting the regeneration frequency to 100% and the maximum value for the remaining output parameters (Table 4). Results provided an optimized dose of 1.0 g/L MS, 0.114 mg/L BAP, and 23.94 g/L sucrose for 100% regeneration frequency, 63.26 shoots with fresh weight of 1.454 g per explant. Investigation of actual and predicted values of output parameters depicted Run 8 as the best combination considering all output parameters. The stated results are very close to Run 8. The experiment performed to validate the results yielded 100.0% regeneration with a 60.68 average shoot count and 1.373 g fresh weight.
Machine learning analysis
The performance of MLP and RF models by employing three different performance metrics (R2, RMSE, and MAE) is presented in Table 5. The performance metrics are used to evaluate the performance of the model. The R2 scores revealed the better performance of the model and vice versa. The analysis of results illustrated the relatively higher R2 scores from the MLP model compared to the RF model for all output parameters used in this study. The results were further analyzed by RMSE and MAE metrics. The scores of RMSE and MAE are generally recorded low for output or model having high R2 scores. Relatively low RMSE and MAE scores were attributed to the MLP model for all parameters. In the next step, actual and predicted scores of all output parameters were expressed as a graphical presentation along with standard 1:1 line in (Fig. 4). The graphical presentation confirmed the results by placing variables with R2 scores near the line and vice versa.
Discussion
In vitro regeneration of aquatic plants is important for clonal propagation with true-to-type plants. The in vitro regeneration protocol can be optimized by manipulating several factors like culture medium which is made of several ingredients like sucrose, basal salts, gelling agent, pH, and plant growth regulators. Basal medium is the mainstay of plant tissue culture, comprised of different micronutrients, macronutrients, and vitamins at different concentrations based on their source. The MS basal salts (Zote et al. 2018), B5 basal salts (Koul et al. 2014), and SH basal salts (Ozcan et al. 2021) are most preferred for in vitro regeneration of aquatic plants. However, the optimum concentration is more important for yielding maximum clonal propagation. The results of the present study revealed high regeneration frequency irrespective of concentration. However, elevated MS concentration in the culture medium led to reduced regeneration frequency, shoot counts per explant and fresh weight. The in vitro regenerated plantlets get their reducing or non-reducing carbon from the presence of sucrose in the culture medium, which also regulates the in vitro morphogenesis process. (Yaseen et al. 2013). The impact of sucrose along with BAP was insignificant in this study.
The optimization of more than two input factors is relatively difficult due to a large number of different combinations with replications in the experiment. The problem can be overcome by adopting statistical tools or programs like response surface methodology (Akin et al. 2017; Poothong and Reed 2014; Reed et al. 2013), which allows to optimization of two or more input factors simultaneously with a relatively low number of combinations (Aghayeh et al. 2020; Ahmadpour et al. 2023). When using the response surface method, the Box-Behnken design is the most popular. It produces desired statistical features lowers the number of component combinations with excellent precision, and functions well during the optimization process. For this purpose, the quadratic model is appropriate for data evaluation because it only requires a small portion of the trials needed for a 3-level factorial (Ahmadpour et al. 2023; Yaghmaeian and Jaafari 2018). However, the appropriate experimental design that will specify which treatments should be carried out in the experimental region under study must be chosen before employing the response surface methodology technique (Yolmeh and Jafari 2017).
Response surface regression analysis is a powerful tool for data analysis, and it allows to investigation of the results in different dimensions using tools offered by the program (Ahmadpour et al. 2023). The RSM offers both the model’s performance and optimization (Kumar et al. 2018). The P value of 0.050 or below reflect the significant results and vice versa more than 0.050 nonsignificant impact. The results revealed that only MS and MS × BAP exerted a significant impact on the in vitro morphogenesis of Brazilian micro sword. The successful use of RSM has been employed for investigating in vitro regeneration or morphogenesis of Dianthus caryophyllus L. (Gutiérrez-Miceli et al. 2010), eggplant (Naveenchandra et al. 2011), Handroanthus impetiginosus (Larraburu et al. 2012), Basilicum polystachyon (Chakraborty et al. 2010), lettuce (Gómez-Montes et al. 2015), Chinese jujube (Hou et al. 2018), soybean (Abbasi et al. 2016), Jatropha curcas (Jose et al. 2019), and seedless barberry (Aghayeh et al. 2020), and Hyoscyamus niger (Ahmadpour et al. 2023). The results of this study revealed variable R2 scores of each output parameter with relatively low predicted R2 scores. The greater difference between actual and predicted R2 scores leads to different predicted scores (Mohamad Thani et al. 2020). Results were also enriched by providing regression model equations for confirmation (Kasman et al. 2019). The response surface regression analysis also benefitted from Pareto charts, normal plots, contour plots, surface plots, and response optimization analysis.
Pareto and Normal plot analysis to investigate the impact of input factors on output parameters in terms of ranking the input factors (Pareto chart) or type of impact (direct or inverse proportional) between inputs and outputs. Results of Pareto charts illustrated the most significant factor for in vitro regeneration. Whereas normal plots displayed the positive impact of AB and negative impact of A factor on shoot counts. Being a powerful tool, both charts have been documented for the production of IAA by plants (Myo et al. 2019) and the biosynthesis of plant-based gold nanoparticles (Keijok et al. 2019). c The results were further analyzed by developing the contour and surface plots. By charting two independent input factors on the x and y axes, both graphical plots are utilized to forecast the output parameters (Younis et al. 2023), and expressed with different colors (Kasman et al. 2019). Contour plots help researchers to determine the best conditions for obtaining the required output parameters by splitting the data into smaller data sets, and they can be further visualized by surface plots that distribute data in three dimensions. All output parameters were optimized using both charts confirmed the use of plots for in vitro regeneration optimization of eggplant (Naveenchandra et al. 2011), Lactuca sativa L. (Gómez-Montes et al. 2015), Jatropha curcas (Jose et al. 2019), Hyoscyamus niger (Ahmadpour et al. 2023), and sorghum (Aasim et al. 2023c). Although both plots are highly efficient for optimization, they are unable to predict multiple factors (three or more) simultaneously. Response optimizer can be used to optimize multiple input factors by adjusting the output parameter as maximum, minimum or desired value (Chaari et al. 2022). Results revealed that optimized inputs were close to run 8. The optimization of all input factors simultaneously is advantageous and it is also possible to get multiple options at the same time. The use of response optimizer is reported for in vitro regeneration of Jatropha curcas (Jose et al. 2019), and Hyoscyamus niger (Ahmadpour et al. 2023).
Because of various dataset constraints, the intricate behavior of in vitro plant tissue culture makes it difficult to explain (Qureshi et al. 2021) and optimize using typical statistical methods (Qureshi et al. 2021). RSM was used to analyze, evaluate, and optimize the data in this study. Furthermore, the data was predicted and validated by putting it into AI/ML-based algorithms. The AI/ML-based algorithms are highly efficient in understanding the data compared to traditional statistical tools (Jafari et al. 2022). However, due to specific challenges and procedures involved in data collecting and processing, the usage of these algorithms is limited (Hesami et al. 2022). The usage of these models has increased gradually in recent years, although their application to aquatic plants remains insubstantial. The use of AI/ML-based models has been documented for in vitro regeneration of Alternantheria reneckii (Aasim et al. 2022), royal purple (Aasim et al. 2013d), and in vitro propagation of aquatic Hemianthus callitrichoides (Cuba) and Riccia fluitans hydrophytes (Özcan et al. 2023). In all of these studies, MLP, RF, and XGBoost models were employed, and results were validated by different performance metrics. The AI/ML models produced comparable findings for all three performance criteria; however, the MLP model predicted and verified the outcomes more precisely than the RF model. Studies on in vitro regeneration comparing MLP and RF models depicted the better performance of the MLP model. Whereas the superior performance of the RF model over MLP is also documented in the literature. It is also established that the performance of each model also varied with the output parameter in the same study (Özcan et al. 2023). Relatively low R2 scores were recorded for shoot length and fresh weight, and satisfactory for shoot counts. The R2 scores reflects the accuracy of the data and depend on the generated dataset. Low R2 scores are thought to be caused by the data produced by the RSM model. However low RMSE and MAE scores for all output parameters confirmed the data validation.
Conclusion
This study demonstrates the successful application of response surface methodology (RSM) for optimizing the input factors for an efficient in vitro regeneration of Brazilian micro sword. Experimentation and data analysis revealed that the best combination was 1.0 g/L MS, 0.1 mg/L BAP, and 25 g/L sucrose. The strong correlation between the expected and actual scores highlights the reliability of the RSM methodology. Furthermore, the use of machine learning techniques strengthened the findings by predicting and validating the data. A comparison of outcomes predicted and confirmed by two different models showed that the MLP model predicted better than the RF model. This study’s outcomes open doors for progress in plant tissue culture, plant biotechnology, and the evolution of complex biological processes. The results signify a new opportunity to incorporate advanced computational modeling techniques for the simultaneous analysis and optimization of multiple variables within the domains of biological sciences and biotechnology.
Data availability
The whole datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANNs:
-
Artificial neural networks
- ANOVA:
-
Analysis of variance
- LOO-CV:
-
Leave-one-out cross validation
- ML:
-
Machine learning
- MLP:
-
Multilayer Perceptron
- MS:
-
Murashige and Skoog Medium
- RF:
-
Random Forest
References
Aasim M, Akin F, Ali SA, Taskin MB, Colak MS, Khawar KM (2023a) Artificial neural network modeling for deciphering the in vitro induced salt stress tolerance in chickpea (Cicer arietinum L). Physiol Mol Biol Plants 1–16. https://doi.org/10.1007/s12298-023-01282-z
Aasim M, Ali SA, Altaf MT, Ali A, Nadeem MA, Baloch FS (2023b) Artificial neural network and decision tree facilitated prediction and validation of cytokinin-auxin induced in vitro organogenesis of sorghum (Sorghum bicolor L.). Plant Cell Tissue Organ Cult 1–14. https://doi.org/10.1007/s11240-023-02498-3
Aasim M, Ali SA, Aydin S, Bakhsh A, Sogukpinar C, Karatas M, Khawar KM, Aydin ME (2023c) Artificial intelligence–based approaches to evaluate and optimize phytoremediation potential of in vitro regenerated aquatic macrophyte Ceratophyllum demersum L. Environ Sci Pollut Res 1–12. https://doi.org/10.1007/s11356-022-25081-3
Aasim M, Ayhan A, Katırcı R, Acar AŞ, Ali SA (2023d) Computing artificial neural network and genetic algorithm for the feature optimization of basal salts and cytokinin-auxin for in vitro organogenesis of royal purple (Cotinus coggygria Scop). Ind Crops Prod 199:116718. https://doi.org/10.1016/j.indcrop.2023.116718
Aasim M, Ali SA, Bekiş P, Nadeem MA (2022) Light-emitting diodes induced in vitro regeneration of Alternanthera reineckii mini and validation via machine learning algorithms. In Vitro Cell Dev Biol 1–10. https://doi.org/10.1007/s11627-022-10312-6
Aasim M, Khawar KM, Ahmed SI, Karataş M (2019) Multiple uses of some important aquatic and semiaquatic medicinal plants. In: Ozturk M, Hakeem K (eds) Plant and Human Health, vol. 2. Springer, pp 541–577. https://doi.org/10.1007/978-3-030-03344-6_23
Abbasi Z, Hooshyar S, Bagherieh-Najjar MB (2016) Improvement of callus production and shoot regeneration using various organs of soybean (Glycine max L. Merr) by response surface methodology. In Vitro Cell Dev Biol 52:537–545. https://doi.org/10.1007/s11627-016-9778-1
Aggarwal CC (2018) Neural networks and deep learning. Springer, Cham. https://doi.org/10.1007/978-3-319-94463-0
Aghayeh RNM, Abedy B, Balandari A, Samiei L, Tehranifar A (2020) Use of response surface methodology for optimizing the media of establishment and proliferation phases of Iranian seedless barberry. Plant Cell Tissue Organ Cult 141:87–101. https://doi.org/10.1007/s11240-020-01769-7
Ahmadpour R, Maleki Zanjani B, Garoosi G, Haddad R, Farjaminezhad R (2023) Prediction of the concentration of plant growth regulators for somatic embryogenesis and regeneration of Hyoscyamus niger using Box–Behnken design of response surface methodology. Plant Cell Tissue Organ Cult 1–17. https://doi.org/10.21203/rs.3.rs-2430673/v1
Akin M, Eyduran E, Reed BM (2017) Use of RSM and CHAID data mining algorithm for predicting mineral nutrition of hazelnut. Plant Cell Tissue Organ Cult. 128:303–316. https://doi.org/10.1007/s11240-016-1125-z
Ali HH, Fayed MIA, Lazim II (2022) Use of aquatic plants in removing pollutants and treating the wastewater: a review. J Glob Innov Agric Sci 10:61–70. https://doi.org/10.22194/JGIAS/10.985
Anderson MJ, Whitcomb PJ (2016) RSM simplified: optimizing processes using response surface methods for design of experiments (2nd ed.). Productivity Press. https://doi.org/10.1201/9781315382326
Aydar AY (2018) Utilization of response surface methodology in optimization of extraction of plant materials. Stat. approaches with Emphas. Des Exp Appl Chem Process 157–169. https://doi.org/10.5772/intechopen.73690
Bone TS, Downie SR, Affolter JM, Spalik K (2011) A phylogenetic and biogeographic study of the genus Lilaeopsis (Apiaceae tribe Oenantheae). Syst Bot 36:789–805. https://doi.org/10.1600/036364411X583745
Chaari M, Elhadef K, Akermi S, Hlima HB, Fourati M, Chakchouk Mtibaa A, Sarkar T, Shariati MA, Rebezov M, D’Amore T (2022) Multiobjective response and chemometric approaches to enhance the phytochemicals and biological activities of beetroot leaves: an unexploited organic waste. Biomass Convers Biorefinery 1–15. https://doi.org/10.1007/s13399-022-03645-0
Chakraborty D, Bandyopadhyay A, Bandopadhyay S, Gupta K, Chatterjee A (2010) Use of response surface methodology for optimization of a shoot regeneration protocol in Basilicum polystachyon. In Vitro Cell Dev Biol 46:451–459. https://doi.org/10.1007/s11627-010-9309-4
Dogan M (2022) High efficiency plant regeneration from shoot tip explants of staurogyne repens (Nees) kuntze. Bangladesh J Bot 51:697–704. https://doi.org/10.3329/bjb.v51i4.63488
Farooq Z, Rehman S, Abid M (2013) Application of response surface methodology to optimize composite flour for the production and enhanced storability of leavened flat bread (Naan). J Food Process Preserv 37:939–945. https://doi.org/10.1111/j.1745-4549.2012.00732.x
Fletcher J, Willby N, Oliver DM, Quilliam RS (2020) Phytoremediation using aquatic plants. In: Shmaefsky B (eds) Phytoremediation. Concepts and Strategies in Plant Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-00099-8_7
Géron A (2022) Hands-on machine learning with Scikit-Learn, Keras, and TensorFlow (2nd ed.). O’Reilly Media, Inc.
Gómez-Montes EO, Oliver-Salvador C, Durán-Figueroa N, Badillo-Corona JA, Salas CE (2015) Optimization of direct shoot regeneration using cotyledonary explants and true leaves from lettuce cv. Romaine (Lactuca sativa L.) by surface response methodology. Plant Growth Regul 77:327–334. https://doi.org/10.1007/s10725-015-0067-5
Gutiérrez-Miceli FA, Arias L, Juarez-Rodríguez N, Abud-Archila M, Amaro-Reyes A, Dendooven L (2010) Optimization of growth regulators and silver nitrate for micropropagation of Dianthus caryophyllus L. with the aid of a response surface experimental design. In Vitro Cell Dev Biol 46:57–63. https://doi.org/10.1007/s11627-009-9259-x
Hesami M, Alizadeh M, Jones AMP, Torkamaneh D (2022) Machine learning: its challenges and opportunities in plant system biology. Appl Microbiol Biotechnol 1–24. https://doi.org/10.1007/s00253-022-11963-6
Hesami M, Jones AMP (2021) Modeling and optimizing callus growth and development in Cannabis sativa using random forest and support vector machine in combination with a genetic algorithm. Appl Microbiol Biotechnol 1–12. https://doi.org/10.1007/s00253-021-11375-y
Hesami M, Naderi R, Tohidfar M, Yoosefzadeh-Najafabadi M (2020) Development of support vector machine-based model and comparative analysis with artificial neural network for modeling the plant tissue culture procedures: effect of plant growth regulators on somatic embryogenesis of chrysanthemum, as a case study. Plant Methods 16:1–15. https://doi.org/10.1186/s13007-020-00655-9
Hou L, Wang Y, Cui Y, Pang X, Li Y (2018) Optimisation of a highly efficient shoot regeneration system using leaf explants of Chinese jujube (Ziziphus jujuba Mill.) by response surface methodology. J Hortic Sci Biotechnol 93:289–295. https://doi.org/10.1080/14620316.2017.1364980
Ing NS, Kharuddin AA, Sahidin N, Zainuddin R, Mahmud NH, Abdullah TA, Ha HC (2019) In vitro micropropagation of aquarium plants pearl grass Hemianthus micranthemoides (Nuttall) and micro sword grass Lilaeopsis brasiliensis (Glaziou) Affolter (Apiaceae). J Agrobiotechnol 10:88–93
Jafari M, Daneshvar MH, Jafari S, Hesami M (2022) Machine Learning-Assisted In Vitro Rooting Optimization in Passiflora caerulea. Forests 13:2020. https://doi.org/10.3390/f13122020
Jamshidi S, Yadollahi A, Arab MM, Soltani M, Eftekhari M, Sabzalipoor H, Sheikhi A, Shiri J (2019) Combining gene expression programming and genetic algorithm as a powerful hybrid modeling approach for pear rootstocks tissue culture media formulation. Plant Methods 15:1–18. https://doi.org/10.1186/s13007-019-0520-y
Kasman M, Riyanti A, Salmariza S, Aslamia RTSS (2019) Response surface methodology approach for analysis of phytoremediation process of Pb (II) from aqueous solution using echinodorus palaefolius. In: IOP conference series: materials science and engineering. IOP Publishing, p 22009. https://doi.org/10.1088/1757-899X/546/2/022009
Karatas M, Dogan M, Emsen B, Aasim M (2015) Determination of in vitro free radical scavenging activities of various extracts from in vitro propagated Ceratophyllum demersum L. Fresenius Environ Bull 24:2946–2952
Katırcı R, Yılmaz EK, Kaynar O, Zontul M (2021) Automated evaluation of Cr-III coated parts using Mask RCNN and ML methods. Surf Coat Technol 422:127571. https://doi.org/10.1016/j.surfcoat.2021.127571
Keijok WJ, Pereira RHA, Alvarez LAC, Prado AR, da Silva AR, Ribeiro J, de Oliveira JP, Guimarães MCC (2019) Controlled biosynthesis of gold nanoparticles with Coffea arabica using factorial design. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-52496-9
Koç B, Kaymak-Ertekin F (2010) Response surface methodology and food processing applications. GIDA-J Food 35:63–70
Koul A, Sharma A, Gupta S, Mallubhotla S (2014) Cost effective protocol for micropropagation of Bacopa monnieri using leaf explants. Int J Sci Res 3:210–212
Kumar V, Singh J, Kumar P (2018) Response surface methodology based optimization of cadmium and lead remediation from aqueous solution by water hyacinth (Eichhornia crassipes [Mart.] Solms) and its anatomical study. Arch Agric Environ Sci 3:163–173. https://doi.org/10.26832/24566632.2018.0302010
Larraburu EE, Apostolo NM, Llorente BE (2012) In vitro propagation of pink lapacho: response surface methodology and factorial analysis for optimisation of medium components. Int J For Res 2012. https://doi.org/10.1155/2012/318258
Mohamad Thani NS, Mohd Ghazi R, Abdul Wahab IR, Mohd Amin MF, Hamzah Z, Nik Yusoff NR (2020) Optimization of phytoremediation of nickel by Alocasia puber using response surface methodology. Water 12:2707. https://doi.org/10.3390/w12102707
Myo EM, Ge B, Ma J, Cui H, Liu B, Shi L, Jiang M, Zhang K (2019) Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol 19:1–14. https://doi.org/10.1186/s12866-019-1528-1
Naveenchandra PM, Bhattacharya S, Ravishankar GA (2011) Culture media optimization through response surface methodology for in vitro shoot bud development of Solanum melongena L. for micropropagation. Int J Bioautom 15:159–172
Oyedeji AA, Abowei JFN (2012) The classification, distribution, control and economic importance of aquatic plants. Int J Fish Aquat Sci 1:118–128
Özcan E, Atar HH, Ali SA, Aasim M (2023) Artificial neural network and decision tree–based models for prediction and validation of in vitro organogenesis of two hydrophytes—Hemianthus callitrichoides and Riccia fluitans. In Vitro Cell Dev Biol 1–16. https://doi.org/10.1007/s11627-023-10367-z
Ozcan E, Onlu S, Sezgin ME, Barpete S (2021) The effect of improvised media and sugar concentration on in vitro shoot multıplıcatıon of Riccıa fluıtans L.: an amphibious liverwort. Fresenıus Environ Bull 30:1696–1702
Pavlov YL (2019) Random forests. Random For 1–122. https://doi.org/10.1201/9780429469275-8
Pepe M, Hesami M, Jones AMP (2021) Machine learning-mediated development and optimization of disinfection protocol and scarification method for ımproved ın vitro germination of cannabis seeds. Plants 10:2397. https://doi.org/10.3390/plants10112397
Poothong S, Reed BM (2014) Modeling the effects of mineral nutrition for improving growth and development of micropropagated red raspberries. Sci Hortic (Amsterdam) 165:132–141. https://doi.org/10.1016/j.scienta.2013.10.040
Qureshi MA, Shafqat W, ud Din S, Raza MK, Sharif N, Ikram S, Rana HAA (2021) Effect of different plant growth regulators on in vitro regeneration of mango: a report. J Glob Innov Agric Sci 9:95–98. https://doi.org/10.22194/JGIAS/9.946
Reed BM, Wada S, DeNoma J, Niedz RP (2013) Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cell Dev Biol 49:343–355. https://doi.org/10.1007/s11627-013-9504-1
Sadat-Hosseini M, Arab MM, Soltani M, Eftekhari M, Soleimani A, Vahdati K (2022) Predictive modeling of Persian walnut (Juglans regia L.) in vitro proliferation media using machine learning approaches: a comparative study of ANN, KNN and GEP models. Plant Methods 18:48. https://doi.org/10.1186/s13007-022-00871-5
Saini DC, Singh SK, Rai K, Singh SK (2010) Biodiversity of aquatic and semi-aquatic plants of Uttar Pradesh: With special reference to eastern Uttar Pradesh. Uttar Pradesh State Biodiversity Board Press
Van Rossum G, Drake FL (2009) Python 3 Reference Manual. CreateSpace, Scotts Valley, CA
Viswanathan P, Gosukonda JS, Sherman SH, Joshee N, Gosukonda RM (2022) Prediction of In vitro organogenesis of Bacopa monnieri using artificial neural networks and regression models. Heliyon 8:e11969. https://doi.org/10.1016/j.heliyon.2022.e11969
Wantala K, Khongkasem E, Khlongkarnpanich N, Sthiannopkao S, Kim K-W (2012) Optimization of As (V) adsorption on Fe-RH-MCM-41-immobilized GAC using Box-Behnken design: effects of pH, loadings, and initial concentrations. Appl Geochem 27:1027–1034. https://doi.org/10.1016/j.apgeochem.2011.11.014
Yaghmaeian K, Jaafari J (2018) Optimization of heavy metal biosorption onto freshwater algae (Chlorella coloniales) algae cells using response surface methodology (RSM). Chemosphere 217:447–455. https://doi.org/10.1016/j.chemosphere.2018.10.205
Yaseen M, Ahmad T, Sablok G, Standardi A, Hafiz IA (2013) Role of carbon sources for in vitro plant growth and development. Mol Biol Rep 40:2837–2849. https://doi.org/10.1007/s11033-012-2299-z
Yolmeh M, Jafari SM (2017) Applications of response surface methodology in the food industry processes. Food Bioprocess Technol 10:413–433. https://doi.org/10.1007/s11947-016-1855-2
Younis M, Ahmed IAM, Ahmed KA, Yehia HM, Abdelkarim DO, Fickak A, El-Abedein AIZ, Alhamdan A, Elfeky A (2023) Pulsed electric field as a novel technology for fresh barhi date shelf-life extension: process optimization using response surface methodology. Horticulturae 9:155. https://doi.org/10.3390/horticulturae9020155
Zote RK, Patil YK, Londhe SS, Thakur VV, Choudhari NB (2018) In vitro regeneration of Bacopa monnieri (L.) from leaf and stem explants. Int J Chem Stud 6:1577–1580
Zurayk R, Sukkariyah B, Baalbaki R (2001) Common hydrophytes as bioindicators of nickel, chromium and cadmium pollution. Water Air Soil Pollut 127:373–388. https://doi.org/10.1023/A:1005209823111
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). There was no financial support to conduct this study.
Author information
Authors and Affiliations
Contributions
SAA: Project administration, Formal analysis, Writing—Review & Editing, Visualization, Data Curation, MA: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original Draft, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest. All authors read the manuscript and showed their willingness to publish the manuscript.
Additional information
Communicated by Saikat Gantait.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, S.A., Aasim, M. Response surface methodology and artificial intelligence modeling for in vitro regeneration of Brazilian micro sword (Lilaeopsis brasiliensis). Plant Cell Tiss Organ Cult 157, 10 (2024). https://doi.org/10.1007/s11240-024-02734-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-024-02734-4