Abstract
This study aimed to determine the effects of 24-epibrasinolide (EBL) and methyl jasmonate (MJ) treatments on growth parameters and secondary metabolite synthesis in adventitious root and cell suspension cultures of Hyoscyamus niger. Therefore, different concentrations (0.5, 1 and 2 mg L−1) of EBL alone and combined with 224.3 mg L−1 (1 mM) MJ were applied to root and cell suspension cultures. 2 mg L−1 and 1 mg L−1 EBL were determined as the treatments in which the highest values were obtained in terms of growth criteria in root and cell cultures, respectively. In root cultures, the highest scopolamine accumulation (2.57 mg g−1) was obtained from the combination of 2 mg L−1 EBL and MJ, while the highest value (0.66 mg g−1) for hyoscyamine was observed in the roots treated with 1 mg L−1 EBL and MJ. In cell cultures, 2 mg L−1 EBL for scopolamine and 0.5 mg L−1 EBL for hyoscyamine were found to be the best applications and calculated as 0.51 µg g−1 and 0.28 µg g−1, respectively. EBL and MJ treatments also stimulated total phenolic content (TPC). The highest TPC in root cultures was detected as 18.01 mg g−1 with the combination of MJ while in cell cultures, maximum TPC was observed in cells applied with 2 mg L−1 EBL and MJ as 11.56 mg g−1. When EBL and MJ were applied to root and cell suspension cultures, significant changes occurred in the amount of phenolic compounds. Co-application of EBL and MJ significantly increased the amount of gallic acid, catechin, epicatechin, cinnamic acid and chlorogenic acid in root cultures. The application of 2 mg L−1 EBL was determined as the most suitable application for gallic acid, catechin, epicatechin, p-coumaric acid, and caffeic acid in cell cultures. It was also found that the metabolite production performance of adventitious roots was higher than that of cells. In conclusion, it was suggested that the use of MJ and EBL may be a promising strategy to enhance the accumulation of scopolamine, hyoscyamine and phenolics in root and cell cultures of H. niger.
Key Message
The concurrent use of MJ and EBL in root and cell cultures had a pronounced impact on both biomass and secondary metabolite production, suggesting their potential for enhancing metabolite yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vitro cultures have been essential tools in plant biotechnology for secondary metabolites production (SMP) used for the manufacturing of drugs, flavors, fragrances, and other high-value products. Medicinal plants are the primary raw materials, especially for the pharmaceutical industry. Almost a quarter of the drugs licensed by the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) originate from plants (Thomford et al. 2018). Today, the demand for secondary metabolites on an industrial scale is mostly met by traditional production methods where plants are collected from their natural habitats. However, the unregulated gathering of plants from their native habitats jeopardizes the survival of these species. This risk is especially higher in plants with root-derived metabolites as they are collected together with their roots. The traditional production system where plants are collected from nature has also many disadvantages such as the inability to obtain metabolites in standard quantity and quality, scarcity of pure and standardized plant material, dependence on the season, and geographical restrictions. To avoid these disadvantages, in vitro secondary metabolite production (SMP) has been considered as an alternative method for obtaining valuable metabolites from plants (Scarpa et al. 2022).
One of the culture techniques used in in vitro SMP is adventitious root cultures. Adventitious roots involve inducing the growth of roots from a plant tissue or cell that would not normally form roots (Rahmat and Kang 2019). Adventitious root cultures are a promising technique for SMP in many plant species, offering several advantages such as rapid growth capabilities, easy extractability, and the potential to produce bioactive compounds in desired quantity and constant quality (Rahmat and Kang 2019). Unlike hairy roots, adventitious roots do not contain bacterial DNA sequences and do not require genetic transformation. Additionally, adventitious root cultures have easy and rapid growth characteristics and the ability to produce stable secondary metabolites (Murthy et al. 2008).
Apart from root cultures, cell suspension culture is another culture technique used for SMP. Cell suspension cultures, which can be established from various plant tissues such as leaves, stems, roots, and embryos, are based on the principle of culturing cells or cell aggregates in a liquid nutrient medium that promotes cell division and growth under sterile and controlled conditions. Cell suspension cultures are highly versatile and influential tools for the synthesis of diverse metabolites, owing to their simplicity and ability to function as miniature factories for metabolite production. Indeed, cell suspension cultures are extensively employed for the synthesis of different valuable metabolites (Whitmer et al. 2003; Zang et al. 2001; Ogata et al. 2014; Çetin and Göktürk Baydar 2016).
The main disadvantage of in vitro SMP is low productivity. Therefore, various approaches can be employed to increase low productivity, such as changing the culture medium and cultural conditions, applying growth regulators, and adding precursors or intermediate metabolites to the culture media (Scarpa et al. 2022). Elicitor applications, which stimulate the enzymes and genes involved in secondary metabolite synthesis, are also one of the methods to increase SMP under in vitro conditions. Methyl jasmonate (MJ) is a signaling molecule that can regulate plant defense responses by its volatile structure and ability to spread through biological membranes. With these properties, MJ is a successful elicitor used for SMP including pharmaceuticals, nutraceuticals, and industrially important compounds in numerous plants (Kim et al. 2007; Wang et al. 2015; Khojasteh et al. 2016; Ibrahim et al. 2021; Rahmati et al. 2023). Brassinosteroids (BRs) are also another plant hormone group used as an elicitor to increase SMP (Aras Aşcı et al. 2019; Demirci et al. 2020). When applied exogenously, BRs play a crucial role in regulating gene expression and activating metabolic pathways, leading to the enhancement of plant growth and SMP (Nolan et al. 2020).
H. niger (black henbane), a member of the Solanaceae family, is a valuable medicinal plant species with high pharmacological activities due to its high content of tropane alkaloids (TAs), particularly scopolamine and hyoscyamine. TAs are among the most commonly used compounds in the formulation of medicines due to their hypnotic, antispasmodic, sedative, and anticholinergic effects (Tytgat 2007; Thawabteh et al. 2019). In addition to TAs, Hyoscyamus niger also contains different groups of secondary metabolites including lignans, coumarinolignans, flavonoids, glycerides, saponins, glycosides, and phenolics (Lunga et al. 2008). H. niger is a highly demanded plant, especially for obtaining TAs. Producing tropane alkaloids (TAs) synthetically is a more costly and challenging process compared to extracting them from plants due to factors such as complex chemical structures and long-variable biosynthetic pathways (Huang et al. 2005). Therefore, it is necessary to obtain TAs from plants, and in vitro SMP techniques offer an important potential to meet the demand for these valuable compounds without harming the natural habitat of the plant.
In this research, the objective was to investigate the influences of MJ and EBL treatment, as external elicitors, on growth parameters and the biosynthesis of TAs and phenolics in adventitious root and cell suspension cultures of H. niger. Besides, the principal component analysis (PCA) was performed to examine the relationship between variables including growth parameters and secondary metabolites determined in the root and cells.
Materials and Methods
Plant materials and seed germination
In this research, adventitious roots and cells obtained from hypocotyls and petioles, respectively, were used as plant materials. Firstly, the seeds of H. niger obtained from the Garden Directorate of Medicinal and Aromatic Plants of Zeytinburnu Municipality, Turkey, were germinated for the obtaining hypocotyls and petioles. To break seed dormancy and increase the germination rate the seeds were soaked in a 250 mg L−1 gibberellic acid (GA3, Sigma-Aldrich, Germany) solution for 48 h. The disinfection process was carried out according to the method of Aljibouri et al. (2012). After surface disinfection, the seeds were placed onto Murashige and Skoog (MS) medium (Murashige and Skoog 1962) enriched with 3% sucrose (Sigma-Aldrich, Germany) and 0.6% agar (Sigma-Aldrich, Germany) and, cultured at 25 ± 1ºC in the dark (Aljibouri et al. 2012). Two weeks after transfer to the culture medium, the seedlings were cultured under the same temperature but with a light period of 16 h light and 8 h dark for another 2 weeks.
Obtaining and propagation of adventitious roots
To form adventitious roots, segments of petioles, approximately 0.5–1 cm in length, were excised from in vitro seedlings and planted in MS medium enriched with 3% sucrose, 0.6% agar, and 2 mg L−1 indole butyric acid (IBA, Sigma-Aldrich, Germany). These cultures were maintained within a dark chamber at a constant temperature of 25 ± 1 °C. After roughly three weeks of cultivation, adventitious roots were isolated from the explants and inoculated on the medium with the identical composition. The cultures were then placed in a shaker set at 90 rpm, operating in darkness at 25 ± 1ºC. Subsequent transfers were conducted three times with 10-day intervals.
Obtaining and propagation of callus
Callus tissues were derived by transferring segments of hypocotyl, approximately 0.5 cm, from in vitro seedlings into MS medium enriched with 3% sucrose, 0.6% agar 0.5 mg L−1 benzyl adenine (BA, Sigma-Aldrich, Germany), and 2 mg L−1 naphthalene acetic acid (NAA, Sigma-Aldrich, Germany). These hypocotyl explants were cultivated under darkness at a stable temperature of 25 ± 1 °C (Aljibouri et al. 2012). The generated calli were subsequently subcultured three times at 4-week intervals, maintaining the same medium and culture conditions.
Elicitor applications
Before application to adventitious root and cell suspension cultures, MJ (Sigma-Aldrich, Germany) and EBL (Phyto Technology Laboratories, USA) stock solutions were prepared using a mixture of 50% ethanol and distilled water, and filter sterilized. EBL was administered to cultures at 0.5, 1, and 2 mg L−1 concentrations either individually or along with 224.3 mg L−1 (1 mM) MJ. As for the control group, a mixture of 50% ethanol and distilled water was added in the same volume as used for EBL and MJ.
Adventitious roots, approximately 1–1.5 g, were transferred into 30 mL of liquid MS medium containing 3% sucrose and 2 mg L−1 IBA. Meanwhile, cell suspensions were established by mixing 1–1.5 g of calli with 30 mL of liquid MS medium enriched with 3% sucrose, 0.5 mg L−1 BA, and 2 mg L−1 NAA. These cultures were incubated in darkness at 25 ± 1ºC while shaking at 100 rpm for 7 days. EBL and MJ were subsequently introduced into the nutrient media in which the 7-day-old adventitious roots and cell suspensions were cultured. Adventitious roots and cells were harvested 2 and 3 weeks after treatments, respectively. After the harvest, adventitious roots and cells were rinsed with sterilized water and utilized for n subsequent analyses. The study was conducted in triplicate, with each replicate consisting of four Erlenmeyer flasks.
Determination of growth parameters
Following the harvest, adventitious roots and cells were weighed and their fresh weights were expressed as g 100 mL−1. Dry weights were determined in g 100 mL−1 after complete drying of roots and cells at 40 °C. The growth indexes of adventitious roots and cells were computed using the equation provided below:
Metabolite extraction in adventitious roots and cells
To determine the levels of TAs and phenolics in adventitious roots and cells, it was employed the extraction method described by Jakabova et al. (2012). Shortly, a 200–250 mg dry, ground sample was extracted three times for 30 min each in 10 mL of methanol: water (3:2) solution using an ultrasonic bath. After removing the solvent using a rotary evaporator, the resulting dry extract was dissolved in methanol and utilized after filtrating.
Determination of TAs by HPLC
The quantification of scopolamine and hyoscyamine alkaloids in roots and cells by HPLC (Shimadzu Corp., Kyoto, Japan) equipped with a diode array detector and an Agilent Eclipse XDB-C18 column (250 mm × 4.6 mm, 5 μm) was done according to the modified method of Boitel-Conti et al. (2000). The flow rate and injection volume of the system were adjusted to 0.8 mL min−1 and 20 μL, respectively, and the column temperature was maintained at 40 °C. HPLC grade 2% acetic acid (A) and 100% methanol (B) were used as mobile phases. The gradient program was set as follows: 0–12 min, 100–88% A; 12–13 min, 88–80% A; 13–33 min, 80–72% A; 33–48 min, 72–70% A. TAs were analyzed at a wavelength of 220 nm. The quantities of hyoscyamine and scopolamine were calculated as mg g−1 in roots and μg g−1 in cells by comparing their peak areas with those of the standards.
Determination of total phenolic content (TPC)
TPC determination in adventitious roots and cells was done according to the Folin-Ciocalteu method (Singleton and Rossi 1965). The absorbance values of the extracts were determined with a spectrophotometer (PG-T70, USA) at 765 nm. TPC in the samples was calculated as mg g−1 of gallic acid equivalent (GAE) using the curve obtained from gallic acid standard.
Determination of phenolic compounds by HPLC
Quantities of phenolics in adventitious roots and cells were determined via HPLC following the method of Göktürk Baydar et al. (2012). The amounts of, catechin, cinnamic acid, o-coumaric acid, epicatechin, gallic acid, rosmarinic acid and vanillin (at 278 nm), p-coumaric acid (at 309 nm), caffeic acid, ferulic acid (at 320 nm), chlorogenic acid (at 325 nm), and rutin and quercetin (at 360 nm) were determined. Each analytical standard was calibrated at its respective wavelength, and the quantities of these compounds in the extracts were expressed in µg g−1 dry weight.
Statistical analyses
The statistical analysis was conducted with the assistance of SPSS 22.0 statistical software, and differences between the applications were determined by Duncan's Multiple Comparison Test at p ≤ 0.05 levels. Principal component analysis (PCA) was conducted in R using the RStudio V4.3.1 environment (15.03.2023; R Studio Team, 2023; R Core Team, 2023 Vienna, Austria). PCA was made with the package ggplot2, factoextra, and FactoMiner from the R environment.
Results
Effects of elicitor implementation on the growth parameters of roots and cells
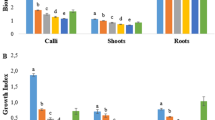
In this study, it was observed that the fresh and dry weights of adventitious roots and cells, as well as growth indexes, showed significant changes based on elicitor implementation (p ≤ 0.05). The research revealed that the application of EBL in roots stimulated root growth and this stimulatory effect increased proportionally with the concentration of EBL (Fig. 1). Compared to the EBL applications, in roots, 224.3 mg L−1 MJ application reduced the root growth. The unfavorable effect of MJ application on root growth was alleviated when combined with EBL applications. The highest fresh and dry root weights and growth index values were detected in the roots administrated with 2 mg L−1 of EBL as 26.10 g 100 mL−1, 1.67 g 100 mL−1, and 3.88, respectively.
Effects of EBL and MJ applications on fresh root weight (A), dry root weight (B), and root growth index (C) in H. niger. (T1:control, T2:0.5 mg L−1 EBL, T3:1 mg L−1 EBL, T4:2 mg L−1 EBL, T5:224.3 mg L−1 MJ, T6: 0.5 mg L−1 EBL + 224.3 mg L−1 MJ, T7:1 mg L−1 EBL + 224.3 mg L−1 MJ, T8: 2 mg L−1 EBL + 224.3 mg L−1 MJ)
EBL treated individually to cell suspension cultures significantly increased the fresh cell weight and cell growth index, particularly when compared to the control and MJ application (Fig. 2). In applications where EBL was used along with MJ, EBL alleviated the unfavorable influence of MJ on the growth of cell. The maximum values in cell growth parameters were obtained from the application of 1 mg L−1 EBL as 5.54 g 100 mL−1 (fresh weight), 0.84 g 100 mL−1 (dry weight), and 0.66 (growth index), respectively.
Effects of EBL and MJ applications on fresh cell weight (A), dry cell weight (B), and cell growth index (C) in H. niger. (T1:control, T2: 0.5 mg L−1 EBL, T3:1 mg L−1 EBL, T4:2 mg L−1 EBL, T5:224.3 mg L−1 MJ, T6: 0.5 mg L−1 EBL + 224.3 mg L−1 MJ, T7:1 mg L−1 EBL + 224.3 mg L−1 MJ, T8: 2 mg L−1 EBL + 224.3 mg L−1 MJ)
Effects of elicitor implementation on the accumulations of hyoscyamine and scopolamine
Applications of EBL at different concentrations, both individually and along with MJ, significantly increase the quantities of hyoscyamine and scopolamine in adventitious roots and cells compared to the control. The greatest amount of hyoscyamine in roots was obtained from the treatment of 1 mg L−1 EBL with MJ (Fig. 3A). The hyoscyamine amount, which was at the lowest level with 0.24 mg g−1 in the control roots, increased to 0.66 mg g−1 with this treatment. When EBL was applied together with MJ, the amounts of scopolamine were found to be higher in adventitious roots than in roots in which EBL and MJ were applied alone. The maximum value for scopolamine with 2.57 mg g−1 was obtained in the roots where 2 mg L−1 EBL was applied together with MJ, and 8 times more scopolamine accumulation was achieved with this application in comparison with the control (Fig. 3B).
The accumulation of hyoscyamine in H. niger cell suspension cultures decreased with the increasing concentrations of EBL applied individually (Fig. 4A). However when applied along with MJ, an increase in EBL concentration enhanced the accumulation of hyoscyamine content. The maximum hyoscyamine concentrations were observed in the applications of 0.5 mg L−1 EBL used alone and in the combined use of 2 mg L−1 EBL with MJ. Scopolamine increased with elicitor applications contrasted with the control. The maximum amount of scopolamine was detected in the cells treated with 2 mg L−1 EBL as 0.51 µg g−1 (Fig. 4B).
Effects of elicitor implementation on the TPC
The utilization of MJ and EBL yielded a substantial enhancement in the TPC within both adventitious roots and cells when contrasted with the control group (Fig. 5A). In the case of adventitious roots, the maximum TPC value of 18.01 mg g−1 was observed in roots treated with MJ while the minimum TPC value was recorded in the control group at 5.91 mg g−1. EBL did have a positive impact on TPC and this beneficial effect was significantly amplified when EBL was introduced in combination with MJ. Elicitors applied to cell suspension cultures led to a significant increase in TPC contrasted with the control. However, the TPC was lower in applications where 0.5 and 1 mg L−1 EBL were used alone when compared with other applications (Fig. 5B). The co-application of 2 mg L−1 EBL with MJ produced the highest TPC at 11.56 mg g−1 in cell suspension cultures.
Effects of elicitor implementation on the phenolic compounds
In this study, it was analyzed that the levels of phenolics including caffeic acid, catechin, chlorogenic acid, cinnamic acid, epicatechin, ferulic acid, gallic acid, o-coumaric acid, p-coumaric acid, rosmarinic acid, rutin, quercetin, and vanillin in the adventitious root and cell cultures of H. niger. While p-coumaric acid, o-coumaric acid, quercetin, and rutin were not detected in adventitious roots, the amounts of other phenolics varied in response to the elicitor treatments (Table 1). The highest gallic acid and cinnamic acid contents were obtained from both MJ and MJ combined with 0.5 and 1 mg L−1 EBL. The combination of EBL with MJ stimulated the production of catechin, and the maximum amount of catechin was obtained from the adventitious roots exposed to the combined treatment of 2 mg L−1 EBL along with MJ. The greatest value in terms of epicatechin was detected in roots implemented with MJ along with EBL at 1 mg L−1 as 93.04 µg g−1. This treatment was followed by the application of 2 mg L−1 EBL and MJ, which resulted in a value of 73.28 µg g−1. While elicitor applications enhanced the content of caffeic acid, rosmarinic acid, and vanillin in the roots, their accumulations reached the maximum levels with the application of MJ. The most effective approach for boosting ferulic acid levels was the use of EBL at 1 mg L−1, leading to a notable elevation in ferulic acid content, reaching 9.61 µg g−1. The roots treated with MJ and MJ in combination with 2 mg L−1 EBL had the greatest levels of chlorogenic acid, which were measured as 4384.66 µg g−1 and 3944.47 µg g−1, respectively, while the control roots exhibited the lowest value. The results indicated that the highest levels of phenolics, except for ferulic acid, were achieved from the adventitious roots implemented with MJ or the combination of EBL and MJ.
While cells did not contain any detectable amount of o-coumaric acid, rutin, and quercetin, it was found that the applications of EBL and MJ significantly altered the levels of detected phenolics (Table 2). The maximum values of catechin, caffeic acid, and gallic acid, were obtained from the cells administrated with 2 mg L−1 of EBL, with levels of 174.88, 17.85, and 28.59 µg g−1, respectively. The amounts of epicatechin in the cells increased in response to all elicitor applications comparison with the control, and the greatest epicatechin levels in cells treated with EBL applications ranged from 707.39 to 780.04 µg g−1. For cell cultures, MJ and 2 mg L−1 EBL were found to be the most suitable applications in enhancing the levels of p-coumaric acid, rosmarinic acid, and vanillin with these applications notable increases were found compared to the control. The highest cinnamic acid value, 5.29 µg g−1, was obtained from the application of 0.5 mg L−1 EBL and MJ combination in contrast, the maximum level of ferulic acid, as 6.96 µg g−1, was observed in the cells implemented with MJ. The most abundant phenolic substance found in cells, similar to adventitious roots, was determined to be chlorogenic acid. The highest amounts of chlorogenic acid, ranging from 2635.25 to 2932.24 µg g−1, were obtained from the cells using EBL and MJ in combination.
Principal component analyses
Principal component analysis (PCA) was used to determine the relationships among all variables including fresh weight, dry weight, growth index, hyoscyamine, scopolamine, TPC, caffeic acid, catechin, chlorogenic acid, cinnamic acid, epicatechin, ferulic acid, gallic acid, p-coumaric acid, rosmarinic acid, and vanillin in root and cells. A two-dimensional scatter plot demonstrating PCA was presented in Fig. 6. The results of PCA indicated that the first three principal components (PCs) had eigenvalues greater than 1.0. Therefore, only the first three PCs were used in the analysis. In roots, the first three PCs described 88.93% of the total data variance, with PC1, PC2, and PC3 representing 47.24%, 26.93%, and 14.76% of the total variance, respectively. The high positive loadings of PC1 were hyoscyamine, TPC, gallic acid, cinnamic acid, rosmarinic acid, caffeic acid, and chlorogenic acid (Table 3). The PC2 involved three variables including fresh root weight, dry root weight, and growth index with high negative loading. This result suggested that roots with high PC2 scores exhibited low growth parameters.
Loading plots of the PCA of variables in eight elicitor applications in root (A) and cell (B) cultures (T1:control, T2:0.5 mg L−1 EBL, T3:1 mg L−1 EBL, T4:2 mg L−1 EBL, T5: 224.3 mg L−1 MJ, T6: 0.5 mg L−1 EBL + 224.3 mg L−1 MJ, T7:1 mg L−1 EBL + 224.3 mg L−1 MJ, T8: 2 mg L−1 EBL + 224.3 mg L−1 MJ and the full names of abbreviated variables are given in Table 3 and 4)
In cells, the results of the PCA were presented in Table 4. The first three PCs explained 89.32% of the total variance. PC1 described 44.50% of the variation, and the greatest factor loads were observed in catechin and epicatechin variables. PC2, representing 32.86% of the total variance, was characterized by high negative loadings of dry weight, fresh weight, and growth index; and high positive loadings of p-coumaric acid, TPC, and ferulic acid.
Discussion
This study aimed to determine the effects of EBL treatments individually or combined with MJ on growth and the syntheses of TAs and phenolics in adventitious root and cell suspension cultures of H. niger. The findings demonstrated that implementing EBL enhanced the growth of both adventitious roots and cells. In adventitious roots, 2 mg L−1 EBL was found to be the most efficient application for promoting growth parameters However, EBL at 1 mg L−1 was the most appropriate implement for cell growth parameters. MJ had no significant effects on the root growth. In cell cultures, MJ showed inhibitory effects on cell growth. However, when MJ was combined with EBL, its inhibitory effect was alleviated.
MJ is known to perform a crucial role in regulating several physiological functions in plants including growth, development, and defense to different stress factors. Studies have shown that MJ treatment can lead to a decrease in root growth. For example, MJ treatments inhibited root growth in Scopolia parviflora (Kkang et al. 2004) and Catharanthus roseus (Ruiz May et al. 2011) MJ treatments also decreased cell proliferation and cell growth in some plant cell cultures (Ruiz May et al. 2011; Çetin and Göktürk Baydar 2016). The repressive influence of MJ on cell growth is due to its ability to prevent the transition from the G1 to the S phase during the mitotic cell cycle, leading to a decrease in the count of actively dividing cells (Patil et al. 2013). Indeed, Noir et al. (2013) reported that the repressive effect of MJ on growth is caused by its negative impact on mitotic division, resulting in a delay in the transition from the mitotic cell cycle to the endoreduplication cycle. Donnez et al. (2011) also attributed the decrease in biomass with MJ applications to the reduction in water and nutrient uptake by cells caused by MJ. Additionally, it is known that MJ-induced growth suppression is also associated to disruptions in mitochondrial membrane integrity as well as reductions in ATP and proteins associated with energy metabolism biosynthesis (Ruiz May et al. 2011). While MJ is often associated with inhibiting growth and development, there was evidence that it had promoting effects on growth parameters as well. Pandey et al. (2022) determined that MJ at concentrations of 1, 2, 3, and 10 μM for 30 days in Valeriana jatamansi root cultures increased the fresh and dry weights of roots. Similarly, 25 μM MJ applied to Carum carvi callus cultures increased the dry cell weight (Rahmati et al. 2023). These results demonstrated that the influence of MJ on growth depended on the genotype, MJ dozes, and treatment duration.
Besides MJ, BRs also have significant involvement in controlling several physiological processes crucial for plant growth and development, including photosynthesis, nutrient absorption, and responses to environmental stress. BRs are also linked to regulating flowering, fruit maturation, and seed germination (Li et al. 2016). Overall, the multifaceted effects of BRs on plant growth and development make them important targets for agricultural applications. This study found that EBL applications promoted both adventitious root and cell growth. Consistent with these findings, EBL treatment increased the fresh and dry weights of Spartina patens cell cultures (Lu et al. 2003) and in hairy cultures of Echinacea purpurea (Demirci et al. 2020). BRs promote growth by enhancing the activity of BRU1 and TCH4 genes, which code for xyloglucan endotransglucosylase (XET) proteins responsible for loosening the cell wall (Fellner 2003). Additionally, it has been reported that BRs promote cell division and expansion, which contribute to growth (Oh and Clouse 1998). According to Hu et al. (2000), BRs enhance cell growth and division by inducing the transcription of cyclin genes, proteins that regulate the progression of the cell cycle, which subsequently stimulate cell division.
In addition to the researches demonstrating the promotion of growth with BR applications, there were also studies showing the negative effects of BRs on growth and development. For instance, Aydın et al. (2006) informed that BR at 0.1, 0.5, and 1 μM to cell cultures of Gossypium hirsitum reduced fresh and dry weights of cells. The negative effects of BRs on growth may be attributed to the BR concentrations. Indeed, BRs at low concentrations promote cell elongation and division, leading to increased growth while at high concentrations, BRs inhibit growth, causing a reduction in cell expansion and division (Müssig et al. 2003). Similarly, Zhang et al. (2021) stated that excessive BR application can lead to stunted growth and reduced photosynthesis. Additionally, BRs implicated in the regulation of senescence and leaf abscission, which can lead to reduced growth and yield (He et al. 1996; Li et al. 2016; Zhang et al. 2021). Hu et al. (2000) found that the dose-dependent effect of BRs on growth may be due to their effect on cell division, which is necessary for increasing the number of cells and ultimately leading to growth. Vukašinović et al. (2021) stated that this inhibitory effect of high BR concentrations on cell division has been attributed to their interference with the function of certain cell cycle regulatory proteins. High concentrations of BRs may interfere with the normal function of these proteins, leading to abnormal cell cycle progression, cell division, and ultimately growth inhibition (Hu et al. 2000; Wang et al. 2002). Therefore, it is important to carefully balance the concentration of BRs to maximize their positive effects on growth while minimizing any potential negative impacts.
In this study, the elicitor treatments were applied to adventitious roots and cell cultures, and the resulting levels of hyoscyamine and scopolamine, the most important TAs in H. niger, were compared with the controls. The results demonstrated a pronounced rise in the accumulation of these alkaloids following the elicitor treatments. The maximum quantities of scopolamine and hyoscyamine in root cultures were detected when MJ was co-applied with 1 and 2 mg L−1 EBL, respectively. As a result of these treatments, hyoscyamine exhibited a 2.75-fold surge, and scopolamine showed a remarkable 8.03-fold boost compared to the control roots. In cell cultures, 2 mg L−1 EBL led to a 1.76-fold increase in scopolamine accumulation when contrasted with the control. Similarly, 0.5 mg L−1 EBL increased hyoscyamine content by 1.87-fold. These findings suggested that the use of MJ and EBL may be a viable strategy for increasing the production of these alkaloids in the roots and cells of H. niger. In a restricted set of research examining the impact of BRs on alkaloid synthesis, it was reported that BRs increased the production of paclitaxel by over 100% in cell suspensions of Taxus chinensis (Zang et al. 2001) and the synthesis of artemisinin by 57% in hairy roots of Artemisia annua (Wang et al. 2002). The stimulatory effect of BRs on metabolite accumulation has been explained by Naeem et al. (2012) resulting from the activation of the genetic potential responsible for SMP by BRs.
In this study, it was found that MJ had stimulating effects on the production of scopolamine and hyoscyamine. Similarly, MJ significantly increased paclitaxel in Taxus canadensis cell cultures (Linden and Phisalaphong 2000), TAs in Scopolia parviflora adventitious root cultures (Kang et al. 2004), and ajmaline, vinblastine, and vincristine alkaloids in Catharantus roseus cell suspension cultures (Ibrahim et al. 2021). Kang et al. (2004) explained that the increase in the synthesis of TAs as a result of MJ applications is due to the upregulation of gene expression responsible for the synthesis of key enzymes putrescine N-methyltransferase (PMT) and hyoscyamine-6β-hydroxylase (H6H) involved in TAs biosynthesis via MJ signaling.
MJ is a key molecule that is involved in the activation of enzymes linked to SMP, and it either directly or indirectly controls the expression of genes related to plant defense mechanisms (Thines et al. 2007). Indeed, Rodriguez et al. (2003) have stated that MJ is a chemical stimulant that stimulates different metabolic reactions and enzyme activities in most cells and roots. The influence of MJ on metabolite accumulation is significantly dose-dependent. Linden and Phisalaphong (2000) observed that the addition of MJ to Taxus canadensis cells results in a notable increase in the accumulation of paclitaxel within the concentration range of 0–200 μM. However, when the concentration of MJ exceeded 200 μM, the accumulation of paclitaxel stopped. MJ functions as a signaling molecule that induces the synthesis of secondary metabolites, at exceedingly low concentrations. However, as the concentration of MJ increases, it can cause cell death, leading to a decrease in metabolite accumulation (Wang et al. 2015). These findings indicate that high concentrations of MJ may have toxic effects on cells, resulting in a decrease in metabolite accumulation.
The effects of elicitor applications on the production of alkaloids as well as total phenolics were investigated in adventitious roots and cell suspension cultures, in this study. Elicitor treatments in both adventitious roots and cells significantly increased the TPC when compared with the controls. In adventitious roots, the maximum TPC was obtained from MJ application, while in cell cultures, MJ together with EBL at 2 mg L−1 yielded the maximum value. Compared to the control, these applications resulted in a 3.08-fold and 2.83-fold increase in TPCs in adventitious roots and cells, respectively. These results demonstrated that EBL and MJ stimulated the synthesis of total phenolics. Demirci et al. (2020) also found that the application of EBL significantly increased the TPC in Echinacea purpurea hairy roots. Similarly, in immobilized Vitis vinifera cells, 0.75 mg L−1 EBL enhanced the TPC by 1.9 times compared to the control (Babalık 2021). In addition to in vitro studies, BR applications under field conditions also increased the phenolic content in Camellia sinensis (Li et al. 2016). The stimulatory impacts of BRs on phenolic contents were linked to the upregulation of genes responsible for the production of enzymes involved in the biosynthesis process (Li et al. 2016).
TPCs in Celastrus paniculatus cell suspension cultures for 72 h rose from 9.30 μg g−1 to 106.82 μg g−1 with MJ application (Anusha et al. 2016). Similarly, MJ increased TPCs in Polygonum multiflorum root cultures (Ho et al. 2018), and cell suspensions of Vitis vinifera (Çetin and Göktürk Baydar 2016). Kim et al. (2007) explained the stimulative influence of MJ on phenolic content as the result of the MJ-inducing phenylalanine ammonia-lyase (PAL) enzyme, leading to the activation of the phenylpropanoid pathway.
In this study, the amounts of some phenolic compounds including caffeic acid, catechin, chlorogenic acid, cinnamic acid, epicatechin, ferulic acid, gallic acid, rosmarinic acid, o-coumaric acid, p-coumaric acid, rutin, quercetin, and vanillin were also investigated. Quercetin, p-coumaric acid, o-coumaric acid, and rutin, in roots; and rutin, o-coumaric acid, and quercetin in cells did not detect. However, the contents of detected phenolics increased with elicitor applications. For ferulic acid, 1 mg L−1 EBL was the best elicitor implement while the maximum values in phenolics except ferulic acid were detected in roots where EBL and MJ were applied together. In combined applications, the concentration of EBL had a notable influence on the synthesis of phenolics. In cell cultures, the combination of MJ and EBL positively affected the production of chlorogenic acid and cinnamic acid. However, the highest values for other compounds were determined in applications where MJ or EBL was used alone. In previous studies, MJ and BR treatments were found to alter the amounts of phenolics. For example, MJ enhanced vanillin accumulation in Capsicum frutescens roots (Suresh and Ravishankar 2005) and Momordica charantia hairy roots (Chung et al. 2016). Giri et al. (2012) stated that gallic acid increased 2.18-fold with 10 µM MJ application in Habenaria edgeworthii callus cultures compared to controls. However, the amount of gallic acid decreased with increasing MJ concentrations, and this compound was not detected in calli treated with MJ concentrations of 200 µM and above. The amounts of rosmarinic acid and caffeic acid in Satureja khuzistanica cell cultures (Khojasteh et al. 2016); rosmarinic acid in cell suspension of Lithospermum erythrorhizon (Ogata et al. 2004) increased with the MJ applications compared to the control. Similarly, Skrzypczak Pietraszek et al. (2014) reported that 100 µM MJ enhanced the chlorogenic acid content sixfold in Exacum affine shoot cultures. In this study, not only MJ but also EBL increased phenolics as reported previously (Xi et al. 2013; Demirci et al. 2020; Babalık 2021). When used at appropriate concentrations, EBL enhanced the amount of caftaric acid, chlorogenic acid, cichoric acid, echinacoside, and p-coumaric acid in hairy roots of Echinacea purpurea (Demirci et al. 2020), and the amount of catechin, chlorogenic acid, epicatechin, gallic acid p-coumaric acid, and quercetin in immobilized Vitis vinifera cells (Babalık 2021). Li et al. (2016) also informed a 15% increase in catechin content compared to the control in Camellia sinensis with 0.1 mg L−1 EBL. The rise in catechin content can be attributed to the heightened activity of phenylalanine ammonia-lyase (PAL) and glutamine oxoglutarate aminotransferase (GOGAT) enzymes, which play a role in catechin biosynthesis stimulated by BRs.
Phenolic compounds are produced via the shikimate pathway from L-phenylalanine and L-tyrosine. Phenylalanine, being the primary amino acid in this biosynthetic pathway, serves as the precursor for the majority of phenolic substances (Santos Sánchez et al. 2019). MJ is recognized for its function as a signaling molecule that activates the expression of genes associated with the PAL enzyme, which is involved in the production of phenolics (Kikowska et al. 2012). Indeed, it was reported that the increase in PAL activity due to the stimulation of the phenylpropanoid pathway by MJ resulted in an enhancement in the synthesis of phenolics in suspension cultures of Coleus blumei (Szabo et al. 1999).
In this study, positive results were obtained in terms of growth parameters and SMP with EBL and MJ applications in both adventitious root and cell suspension cultures. However, adventitious root cultures showed a higher metabolite production performance compared to cell suspension cultures. Similarly, it has been found that root cultures of medicinally important plants such as Andrographis paniculata (Praveen et al. 2009) and Polygonum multiflorum (Ho et al. 2018) were more effective in terms of biomass increase and secondary metabolite yield compared to cell suspension cultures. Elicitation applications may be used to increase SMP in undifferentiated cultures such as cell suspensions. However, compared to differentiated cultures like root cultures, metabolite production is lower in undifferentiated cultures due to low productivity, genetic and biosynthetic instability over a long culture period, and less efficient response to the same elicitor (Halder et al. 2019). Especially, in undifferentiated cultures, genetic and physical instability can result in a decline or even the depletion of bioactive compounds as time passes (Whitmer et al. 2003). In addition, undifferentiated cells lack storage tissue where metabolites can accumulate. Under these circumstances, metabolites that cannot be stored within the cells and are instead released into the culture medium are susceptible to degradation by enzymes in the culture medium (Cai et al. 2012). Differentiated cultures are effectively used in the production of many valuable metabolites with their genetic and biosynthetic stability and rapid growth ability. For example, Wang and Wu (2010) reported that root cultures were more effective than cell cultures in terms of tanshinone production in Salvia miltiorrhiza. Murthy et al. (2014) also stated that the amount of hypericin in Hypericum perforatum roots (1.38 mg g−1) was more than in the cells (0.16 mg g−1). Similarly, in Eryngium planum, roots had more phenolic acids including caffeic acid, chlorogenic acid and rosmarinic acid, compared to cells (Kikowska et al. 2012). The results, which revealed that differentiated tissues provide higher metabolite production than undifferentiated tissues, agree with the presented study’s results.
PCA is a powerful technique for dimensionality reduction in a dataset, achieved by explaining the variance of interrelated variables. Reducing the dimensionality of data is a useful approach to improve the visualization of data structure. In this study, the first three PCs were selected for PC analyses. The selection of PCs was made according to the Kaiser criterion, which prescribes the selection of PCs with eigenvalues greater than one (Kaiser 1958). The PC1 had the highest eigenvalues and accounted for the largest portion of the variance in the dataset. TPC analyses provided information on the relationships among variables and which components influence the variables. In this study, it was determined that hyoscyamine, TPC, gallic acid, cinnamic acid, rosmarinic acid, caffeic acid, and chlorogenic acid correlated with PC1 in the roots; and scopolamine, gallic acid, catechin, and epicatechin correlated PC1 in the cells.
Conclusion
In this study, EBL applied single or in combination with 224.3 mg L−1 MJ to adventitious root and cell suspension cultures of H. niger altered growth and SMP depending on its concentrations. MJ had a suppressive effect on growth in both roots and cells, whereas EBL treatments promoted growth properties. It was found that all treatments increased the accumulation of TAs and phenolics compared to the control, but this increasing effect varied depending on the concentrations. It was also determined that the metabolite production performance of adventitious roots was higher than that of cells. These findings suggest that the use of MJ and EBL could be a promising strategy for enhancing the accumulation of TAs and phenolic compounds in both root and cell cultures of H. niger.
Data availability
All data generated or analysed during this study are included in this article.
References
Aljibouri AMJ, Al Samarraei KW, Abd AS, Mageed DM, Ali AJA (2012) Alkaloids production from callus of Hyoscyamus niger L. in vitro. J Life Sci 6(8):874
Anusha TS, Joseph MV, Elyas KK (2016) Callus induction and elicitation of total phenolics in callus cell suspension culture of Celastrus paniculatus–willd, an endangered medicinal plant in India. Pharmacognosy J 8(5):471–475. https://doi.org/10.5530/pj.2016.5.10
Aras Aşcı Ö, Deveci H, Erdeğer A, Özdemir KN, Demirci T, Göktürk Baydar N (2019) Brassinosteroids promote growth and secondary metabolite production in Lavandin (Lavandula intermedia Emeric ex Loisel.). J Essent Oil Res 22:254–263. https://doi.org/10.1080/0972060X.2019.1585298
Aydın Y, Talas Ogras T, Ipekçi Altas Z, Gozukirmizi N (2006) Effects of brassinosteroid on cotton regeneration via somatic embryogenesis. Biologia 61(3):289–293. https://doi.org/10.2478/s11756-006-0053-5
Babalık Z (2021) Increasing of phenolic compounds by brassinosteroid applications in immobilized cell suspension cultures of Vitis vinifera L. cv. Cinsault. J Agric Sci 27(3):298–303. https://doi.org/10.15832/ankutbd.674860
Boitel Conti M, Laberche JC, Lanoue A, Ducrocq C, Sangwan Norreel BS (2000) Influence of feeding precursors on tropane alkaloid production during an abiotic stress in Datura innoxia transformed roots. Plant Cell, Tissue Organ Cult 60(2):131–137. https://doi.org/10.1023/A:1006426314274
Cai Z, Kastell A, Knorr D, Smetanska I (2012) Exudation: an expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep 31:461–477. https://doi.org/10.1007/s00299-011-1165-0
Çetin ES, Göktürk Baydar N (2016) Elicitor applications to cell suspension culture for production of phenolic compounds in grapevine. J Agric Sci 22(1):42–53. https://doi.org/10.1501/Tarimbil_0000001366
Chung IM, Thiruvengadam M, Rekha K, Rajakumar G (2016) Elicitation enhanced the production of phenolic compounds and biological activities in hairy root cultures of bitter melon (Momordica charantia L.). Brazilian Archives of Biology and Technology 59. https://doi.org/10.1590/1678-4324-2016160393
Demirci T, Çelikkol Akçay U, Göktürk Baydar N (2020) Effects of 24-epibrassinolide and l-phenylalanine on growth and caffeic acid derivative production in hairy root culture of Echinacea purpurea L. Moench Acta Physiologiae Plantarum 42:1–11. https://doi.org/10.1007/s11738-020-03055-7
Donnez D, Kim KH, Antoine S, Conreux A, De Luca V, Jeandet P, Courot E (2011) Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2 L stirred bioreactor. Process Biochem 46(5):1056–1062. https://doi.org/10.1016/j.procbio.2011.01.019
Fellner M (2003) Recent progress in brassinosteroid research: hormone perception and signal transduction. In: Hayat S, Ahmad A (eds) Brassinosteroids: bioactivity and crop productivity, 1st edn. Springer, Dordrecht, pp 69–86
Giri L, Dhyani P, Rawat S, Bhatt ID, Nandi SK, Rawal RS, Pande V (2012) In vitro production of phenolic compounds and antioxidant activity in callus suspension cultures of Habenaria edgeworthii: A rare Himalayan medicinal orchid. Ind Crops Prod 39:1–6. https://doi.org/10.1016/j.indcrop.2012.01.024
Göktürk Baydar N, Babalık Z, Türk F, Çetin ES (2012) Phenolic composition and antioxidant activities of wines and extracts of some grape varieties grown in Turkey. J Agric Sci 17:67–76. https://doi.org/10.1501/Tarimbil_0000001157
Halder M, Sarkar S, Jha S (2019) Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci 19(12):880–895. https://doi.org/10.1002/elsc.201900058
He Y, Xu R, Zhao Y (1996) Enhancement of senescence by epibrassinolide in leaves of mung bean seedling. Acta Phytophysiol Sin 22:58–62
Ho TT, Lee JD, Ahn MS, Kim SW, Park SY (2018) Enhanced production of phenolic compounds in hairy root cultures of Polygonum multiflorum and its metabolite discrimination using HPLC and FT-IR methods. Appl Microbiol Biotechnol 102(22):9563–9575. https://doi.org/10.1007/s00253-018-9359-9
Hu Y, Bao F, Li J (2000) Promotive effect of brassinosteroids on cell division involves a distinct cycd3- induction pathway in Arabidopsis. Plant J 24(5):693–701. https://doi.org/10.1046/j.1365-313x.2000.00915.x
Ibrahim MM, Danial N, Matter MA, Rady MR (2021) Effect of light and methyl jasmonate on the accumulation of anticancer compounds in cell suspension cultures of Catharanthus roseus. Egypt Pharm J 20(4):294–302. https://doi.org/10.4103/epj.epj_48_21
Jakabova S, Vincze L, Farkas Á, Kilár F, Boros B, Felinger A (2012) Determination of tropane alkaloids atropine and scopolamine by liquid chromatography–mass spectrometry in plant organs of Datura species. J. Chromatogr. A, Selected Papers from the 36th International Symposium on High-Performance Liquid Phase Separations and Related Techniques 1232: 295–301. https://doi.org/10.1016/j.chroma.2012.02.036
Kaiser HF (1958) The varimax criterion for analytic rotation in factor analysis. Psychometrika 23:187–200. https://doi.org/10.1007/BF02289233
Kang SM, Jung HY, Kanga YM, Yun DJ, Bahk JD, Yang JK, Choi MS (2004) Effects of methyl jasmonate and salicylic acid on the production of tropane alkaloids and the expression of pmt and h6h in adventitious root cultures of Scopolia parviflora. Plant Sci 166:745–751. https://doi.org/10.1016/j.plantsci.2003.11.022
Khojasteh A, Mirjalili MH, Palazon J, Eibl R, Cusido RM (2016) Methyl jasmonate enhanced production of rosmarinic acid in cell cultures of Satureja khuzistanica in a bioreactor. Eng Life Sci 16(8):740–749. https://doi.org/10.1002/elsc.201600064
Kikowska M, Budzianowski J, Krawczyk A, Thiem B (2012) Accumulation of rosmarinic, chlorogenic and caffeic acids in in vitro cultures of Eryngium planum L. Acta Physiol Plant 34:2425–2433. https://doi.org/10.1007/s11738-012-1011-1
Kim HJ, Fonseca JM, Choi JH, Kubota C (2007) Effect of methyl jasmonate on phenolic compounds and carotenoids of romaine lettuce (Lactuca sativa L.). J Agric Food Chem 55(25):10366–10372. https://doi.org/10.1021/jf071927m
Li X, Ahammed GJ, Li ZX, Zhang L, Wei JP, Shen C, Han WY (2016) Brassinosteroids improve quality of summer tea (Camellia sinensis L.) by balancing biosynthesis of polyphenols and amino acids. Front Plant Sci 7:1304. https://doi.org/10.3389/fpls.2016.01304
Linden JC, Phisalaphong M (2000) Oligosaccharides potentiate methyl jasmonate-induced production of paclitaxel in Taxus canadensis. Plant Sci 158(1–2):41–51. https://doi.org/10.1016/S0168-9452(00)00306-X
Lu Z, Huang M, Ge DP, Yang YH, Cai XN, Qin P, She JM (2003) Effect of brassinolide on callus growth and regeneration in Spartina patens (Poaceae). Plant Cell, Tissue Organ Cult 73:87–89. https://doi.org/10.1023/A:1022665210113
Lunga IP, Kintia SS, Bassarella S, Pizzat C (2008) Steroidal saponins from the seeds of Hyoscyamus niger. Chem J Moldova 3:89–93. https://doi.org/10.19261/cjm.2008.03(1).10
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Murthy HN, Hahn EJ, Paek KY (2008) Adventitious roots and secondary metabolism. Chin J Biotechnol 24(5):711–716. https://doi.org/10.1016/S1872-2075(08)60035-7
Naeem M, Idrees M, Alam MM, Aftab T, Khan MMA (2012) Brassinosteroid-mediated enrichment in yield attributes, active constituents and essential oil production in Mentha arvensis L. Russ Agric Sci 38(2):106–113. https://doi.org/10.3103/S1068367412020176
Noir S, Bömer M, Takahashi N, Ishida T, Tsui TL, Balbi V, Devoto A (2013) Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol 161(4):1930–1951. https://doi.org/10.1104/pp.113.214908
Nolan TM, Vukašinović N, Liu D, Russinova E, Yin Y (2020) Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32(2):295–318. https://doi.org/10.1105/tpc.19.00335
Ogata A, Tsuruga A, Matsuno M, Mizukami H (2004) Elicitor-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures: Activities of rosmarinic acid synthase and the final two cytochrome P450-catalyzed hydroxylations. Plant Biotechnol 21(5):393–396. https://doi.org/10.5511/plantbiotechnology.21.393
Oh MH, Clouse SD (1998) Brassinolide affects the rate of cell division in isolated leaf protoplasts of Petunia hybrida. Plant Cell Rep 17:921–924. https://doi.org/10.1007/s002990050510
Pandey S, Sundararajan S, Ramalingam S, Pant B (2022) Elicitation and plant growth hormone-mediated adventitious root cultures for enhanced valepotriates accumulation in commercially important medicinal plant Valeriana jatamansi Jones. Acta Physiol Plant 44:1–13. https://doi.org/10.1007/s11738-021-03319-w
Patil AD, Patil AY, Raje AA (2013) Antidepressant like property of Hyoscyamus niger Linn. in mouse model of depression. Innov Pharm Pharmacother 1(2):60–69
Praveen N, Manohar SH, Naik PM, Nayeem A, Jeong JH, Murthy HN (2009) Production of andrographolide from adventitious root cultures of Andrographis paniculata. Curr Sci 96(5):694–697
Rahmat E, Kang Y (2019) Adventitious root culture for secondary metabolite production in medicinal plants: a review. J Plant Biotechnol 46(3):143–157. https://doi.org/10.5010/JPB.2019.46.3.143
Rahmati M, Golkar P, Tarkesh M (2023) Effects of methyl jasmonate elicitation on the carvone and limonene contents, phenolic compounds and antioxidant activity in caraway (Carum carvi L.) callus cultures. Nat Product Res 1–6. https://doi.org/10.1080/14786419.2023.2169862
Rodriguez S, Compagnon V, Crouch NP, St Pierre B, De Luca V (2003) Jasmonate-induced epoxidation of tabersonine by a cytochrome P-450 in hairy root cultures of Catharanthus roseus. Phytochemistry 64(2):401–409. https://doi.org/10.1016/S0031-9422(03)00269-3
Ruiz May E, De la Pena C, Galaz Avalos RM, Lei Z, Watson BS, Sumner LW, LoyolaVargas VM (2011) Methyl jasmonate induces ATP biosynthesis deficiency and accumulation of proteins related to secondary metabolism in Catharanthus roseus (L.) G. hairy roots. Plant Cell Physiol 52(8):1401–1421. https://doi.org/10.1093/pcp/pcr086
Santos Sánchez NF, Salas Coronado R, Hernández Carlos B, Villanueva Cañongo C (2019) Shikimic acid pathway in biosynthesis of phenolic compounds. Plant Physiol Aspects Phenolic Compounds 1:1–15. https://doi.org/10.5772/intechopen.83815
Scarpa GM, Prota V, Schianchi N, Manunta F (2022) In vitro cultures for the production of secondary metabolites. In Secondary Metabolites IntechOpen.https://doi.org/10.5772/intechopen.101880
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–158. https://doi.org/10.12691/jfnr-2-8-11
Skrzypczak Pietraszek E, Słota J, Pietraszek J (2014) The influence of L-phenylalanine, methyl jasmonate and sucrose concentration on the accumulation of phenolic acids in Exacum affine Balf. f. ex Regel shoot culture. Acta Biochim Polonica 61(1):47–53
Suresh B, Ravishankar GA (2005) Methyl jasmonate modulated biotransformation of phenylpropanoids to vanillin related metabolites using Capsicum frutescens root cultures. Plant Physiol Biochem 43(2):125–131. https://doi.org/10.1016/j.plaphy.2005.01.006
Szabo E, Thelen A, Petersen M (1999) Fungal elicitor preparations and methyl jasmonate enhance rosmarinic acid accumulation in suspension cultures of Coleus blumei. Plant Cell Rep 18:485–489. https://doi.org/10.1007/s002990050608
Thawabteh A, Juma S, Bader M, Karaman D, Scrano L, Bufo SA, Karaman R (2019) The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 11(11):656. https://doi.org/10.3390/toxins11110656
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Howe GA (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448(7154):661–665. https://doi.org/10.1038/nature05960
Thomford NE, Senthebane DA, Rowe A, Munro D, Seele P, Maroyi A, Dzobo K (2018) Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int J Mol Sci 19(6):1578. https://doi.org/10.3390/ijms19061578
Tytgat GN (2007) Hyoscine butylbromide: a review of its use in the treatment of abdominal cramping and pain. Drugs 67:1343–1357. https://doi.org/10.2165/00003495-200767090-00007
Vukašinović N, Wang Y, Vanhoutte I, Fendrych M, Guo B, Kvasnica M, Jiroutová P, Oklestkova J, Strnad M, Russinova E (2021) Local brassinosteroid biosynthesis enables optimal root growth. Nat Plants 7:619–632. https://doi.org/10.1038/s41477-021-00917-x
Wang JW, Wu JY (2010) Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures. Appl Microbiol Biotechnol 88(2):437–449. https://doi.org/10.1007/s00253-010-2797-7
Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, Chory J (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2(4):505–513. https://doi.org/10.1016/s1534-5807(02)00153-3
Wang J, Qian J, Yao L, Lu Y (2015) Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour Bioprocess 2(1):1–9. https://doi.org/10.1186/s40643-014-0033-5
Whitmer S, Canel C, Van Der Heijden R, Verpoorte R (2003) Long-term instability of alkaloid production by stably transformed cell lines of Catharanthus roseus. Plant Cell, Tissue Organ Cult 74:73–80. https://doi.org/10.1023/A:1023368309831
Zang X, Mei XG, Zhang CH, Lu CT, Ke T (2001) Improved paclitaxel accumulation in cell suspension cultures of Taxus chinensis by brassinolide. Biotech Lett 23:1047–1049. https://doi.org/10.1023/A:1010589304749
Zhang X, Guo X, Wang Y, Ma X (2021) Brassinosteroids: biosynthesis, metabolism, and biotechnological applications. Plant Cell Rep 40(1):17–35
Acknowledgements
The authors are thankful to Isparta University of Applied Sciences, Scientific Research Projects Coordination Unit for the financial support for this research Project (Project number: 2020-BTAB-0082).
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
IA: Conceptualization, Methodology, Writing – original draft. TD: Conceptualization, Methodology, Formal analysis, HPLC analysis, Data curation, Writing – review & editing, NGB: Conceptualization, design, Writing – review & editing, Supervision, Project administration, Funding acquisition. All authors contributed to the writing and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no competing interests.
Additional information
Communicated by Pamela J. Weathers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albayrak, İ., Demirci, T. & Baydar, N.G. Enhancement of in vitro production of tropane alkaloids and phenolic compounds in Hyoscyamus niger by culture types and elicitor treatments. Plant Cell Tiss Organ Cult 156, 72 (2024). https://doi.org/10.1007/s11240-024-02692-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-024-02692-x