Abstract
Since the 1980s, there has been a significant emphasis in biotechnology on the utilisation of medicinal plants as a source of raw materials for the pharmaceutical industry. Moreover, medicinal plants have been identified as a potential alternative source of essential compounds with a wide range of applications, including those involved in producing medications, nutraceuticals, food additives, cosmeceuticals, natural pigments, and preservatives. The plant micropropagation system is an ideal solution to the problems caused by conventional exploitation since it may simultaneously provide uniform biomass as a source of bioactive secondary metabolites and ex situ conservation of uncommon or endangered plant species (dual strategies of micropropagation). In honour of the Polish Botany Society’s Centenary anniversary in 2022, this article summarises the development of an in vitro propagation method for selected medicinal plants by Polish researchers and botanists.
Key Message
This article summarises the development of an in vitro propagation and shoot cultures for selected medicinal plants by Polish researchers and botanists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apart from high scientific values, plant biotechnology possesses great practical importance. One of the advantages is using in vitro cultures of medicinal plants to obtain valuable secondary metabolites, which is possible thanks to the collaboration of biologists, biochemists, and technologists. The result of their study is the development of protocols for the rapid, efficient in vitro propagation of several medicinal plant species. With the simultaneous shrinkage of their natural resources, the increasing demand for medicinal plants stimulates the further development of fast reproduction methods to obtain valuable raw materials with increased biomass and a higher content of desirable secondary metabolites (Moraes et al. 2021).
Since the 1980s, medicinal plants have been of great interest in biotechnology as an essential source of raw material for the pharmaceutical industry. Nevertheless, it is estimated that only a fraction per 250,000–500,000 plant species have been scientifically investigated for their biological activity (Ngo et al. 2013). Within these species, only 15,000 of all estimated species have been documented for medicinal use (Wawrosch and Zotchev 2021). The advantages of micropropagation of various medicinal plant species have been described by numerous authors during the last four decades and reviewed in several publications (Matkowski 2008; Smetanska 2008; Moraes et al. 2021). Moreover, methods for in vitro mass propagation of selected taxa or multiplication and conservation of endangered species have been described (Murch et al. 2004; Rybczyński and Mikuła 2006; Thiem et al. 2008; Pence 2010; Tasheva and Kosturkova 2013).
The Polish Botany Society celebrates its 100th anniversary in 2022. To commemorate this occasion, we have prepared this study, which focuses on the micropropagation protocol development of chosen medicinal plants established by Polish botanists in Poland. A summary history of 65 years of plant in vitro techniques development in Poland has been described previously by Zenkteler and Zenkteler (2013). The achievements of Polish researchers in the horticultural and crop, fruits and vegetables, and ornamental plant micropropagation resulted from long-term studies in the Institute of Horticulture Research in Skierniewice (Poland) and described by Podwyszyńska et al. (2022). At the same time, reviews on Polish researchers’ efforts to use in vitro culture to understand the plant developmental process at the cellular level (Płażek and Dubert 2022) and bioactive compounds production via the application of various biotechnological methods, including cell and organ cultures obtained from the cultures (Pietrosiuk et al. 2022) has been described previously.
Over the last century, many institutions and research laboratories in Poland engaged in developing micropropagation techniques for medicinal plants or plants without prior knowledge of medicinal importance. These studies have been reported within national and international scope. In this paper, we collected correlative references through the following databases: PubMed, Google Scholar, and the internal library of Poznan University of Medical Sciences (Poznan, Poland). This study aims to summarise the achievements of Polish researchers in developing in vitro propagation protocols for selected medicinal plants and potential taxa with medicinal value. A brief review of shoot cultures research, which are multiplicated as biomass for phytochemical analyses of bioactive metabolites, are also included.
Medicinal plant micropropagation system and its importance
Plants are the source of valuable substances which can be used as pharmaceuticals, nutraceuticals, food additives, cosmeceuticals, natural pigments, and preservatives. Medicinal plant species are still in great demand and play an important role in the global healthcare system. It is an essential component in herbal medicine research development. Plant secondary metabolites are utilised as a source of natural drugs (drugs of natural origin, e.g., atropine, scopolamine), raw material for semi-synthetic chemical compounds (e.g., irinotecan from camptothecin), a model for novel synthetic chemicals (e.g., cocaine for procaine), and taxonomic markers for discovering new compounds (Balunas and Kinghorn 2005; Debnath et al. 2006). Additionally, the complexity of desired products often makes it impractical and necessitates intricate multistage protocols for synthetic production. This significantly raises production costs and renders profitability uncertain. On the other hand, high demand for medicinal plants often results in habitat degradation, over-exploitation, and local species extinction because there is no control over their harvesting from the wild. The in vitro propagation system offers an integrated system of both ex situ conservation and biomass supply as a source of bioactive chemicals to resolve these concerns (Thiem et al. 2008; Tasheva and Kosturkova 2013; Moraes et al. 2021).
Some potential medicinal plants are rare, endangered or protected by the law, so their biomass is either difficult to harvest from natural habitats or inaccessible. However, these plants’ bioactive compounds, often used in traditional medicine, remain unexplored. The unknown species often possess novel bioactive natural products with potential value. As an example, for the first time, the presence of iridoids and triterpenoid saponins was demonstrated in the biomass obtained from in vitro micropropagated Linnaea borealis, the protected species in Poland, or phenolic compounds in clonal micropropagated Eryngium alpinum, endangered and protected taxa in Europe. In vitro propagation allows large biomass production without causing further damage to the naturally grown species and at the same time, allows for the initial identification of chemical profiles (e.g., L. borealis, E. alpinum, E. maritimum). The experiments on the rare and endangered species in Poland are tightly controlled and require special permission from the Ministry of Climate and Environment (Kikowska et al. 2014; Thiem et al. 2021).
Plant micropropagation is a process that uses in vitro technology to mass-produce valuable plant material, crop, and active ingredients. The aseptic conditions and suitable media in in vitro propagation gave more advantages than the conventional method, especially for plants with medicinal values. Traditional plant propagation is often time and labour-consuming, ineffective in many species, and does not guarantee genetic homogeneity. In vitro propagation for plants is independent of climatic factors and seasons. Therefore, it allows rapid multiplication of plants with recalcitrant or without seeds and constantly provides plant biomass. The advantages of micropropagation from various medicinal plant species have been described by numerous authors and reviewed in several publications (Bajaj et al. 1988; Rout et al. 2000; Karuppusamy 2009). The micropropagation approach also offers an integrated strategy for mass production while fulfilling the Good Laboratory Practice (GLP) and Good Manufacture Practice (GMP) to obtain better secondary metabolites output with continuous production monitoring (Matkowski 2008; Moraes et al. 2021). From the pharmaceutical point of view, the application of micropropagation methods for medicinal plants gives several benefits: acceleration of multiplication rate, uniform clones of high-yielding genotype, conservation of genetic resources, disease-free plant material, biosynthesis of novel compounds, and the identification of elite phenotypes using bioassay as a selection tool (Moraes et al. 2021).
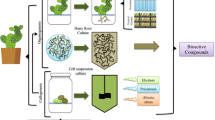
The European Scientific Cooperation for Phytotherapy (ESCOP) commissions highlighted that the production of in vitro plant materials should be standardised and follow the GLP and GMP recommendations. Material control and quality assessment are essential throughout the process to eliminate the risk of abnormality and losing desired phytochemical properties (Purohit et al. 2011). The multiplied microplants or microshoots should undergo morphological, genetic, physiological, biochemical, and phytochemical evaluation (Fig. 1).
First, morphology assessment assures the phenotypic true-to-type feature of the multiplied microshoots. The interaction between the cultivated plants, cultured media composition, plant metabolism, and environmental conditions in vitro affected the plant’s physiological properties. Therefore, the obtained biomass has no physiological and developmental problems, such as necrosis of the explant, hyperhydricity, fasciation, or somaclonal variations (Isah 2015). Secondly, physiology assessment evaluates the essential ability of obtained plantlets to carry out the photosynthesis process and the correct operation of the stomata. Assessment of morphology can be performed non-invasively using video cameras. The technology allows analysing stomata movement, therefore indirectly in the measurements of transpiration and photosynthesis. The results between conventional propagation and the in vitro technique could indicate the existence of differences in the structure and functions of stomata. In plants obtained from in vitro propagation, the closing of stomata in response to external stimuli is slower, which makes it necessary to maintain high humidity during acclimatisation. Photosynthesis is the physiological marker of the obtained in vitro culture. Its efficiency is assessed based on the relationship between the amount of absorbed quanta, the assimilation of one molecule of CO2, and the production of dry mass (Bach and Pawłowska 2009). Thirdly, the genetic variations that may occur during long-term cultivation are evaluated through cytogenetic and molecular analysis. Detection of possible variations through molecular analysis can help to assure the genetic fidelity in regenerated microshoots. Flow cytometry for nuclear DNA content estimation or molecular markers detection such as restriction fragment length polymorphisms (RFLPs), randomly amplified polymorphic DNAs (RAPDs), amplified fragment length polymorphisms (AFLPs), simple sequence repeat (SSRs), and inter simple sequence repeat (ISSR) have been applied to study and identified genetic changes in micropropagated plants (Sliwinska and Thiem 2007; Tyagi et al. 2021). Biochemical and phytochemical measurement is essential to calculate future pharmaceutical raw material devaluation. Using one- and two-dimensional thin-layer chromatography, it is possible to initially control whether the ability to biosynthesis of selected secondary metabolites is preserved in the cultivated organs and regenerated plants. Mass spectrometry techniques are resourceful in detecting changes in the metabolite production or bioactive substances of the in vitro biomass (Chun et al. 2020). Lastly, microbiological evaluation is necessary to mitigate contamination risk that can lead to spurious experimental results arising from latent contaminants or the loss of valuable experiments or commercial culture (Cassells 2012).
Recently, there has been a growing demand that plantlets produced in in vitro cultures laboratories should be certified. Propagated material for commercial purposes obtained by micropropagation should be genetically stable and pathogen-free, as documented by health certification. The certificate should include information about the high quality of the propagation product as well as the international standards-compliant manufacturing method. Such plantlets should meet the quality standard of the genotype and be in the correct physiological condition, guaranteeing adaptation to further development in vivo. Furthermore, in the case of medicinal plants, the phytochemical evaluation should demonstrate the microplants’ ability to biosynthesise biologically active chemicals at a level equivalent to plants acquired using conventional procedures (van der Linde 2000).
In vitro propagation of medicinal plants in Poland
Poland is one of the prominent exporters of plant-derived medicines. According to the average amount of exported medicinal and aromatic plants between 2010 and 2019, Poland is among the top 15 exporters. This data emphasises the relevance of Polish medicinal plants in the global market (Sucholas et al. 2021).
University centres and scientific institutes have noticed the usage of plant biotechnology in the field of plant in vitro cultures. Among them are Prof. Jerzy Czosnowski and Dr. Marian Zieliński from the General Botany Department of Poznan University. They visited Roger Gautheret’s plant biology laboratory and learnt about plant in vitro techniques. Upon their return, they were the first scientists applying the newly acquired knowledge in Polish laboratory practice (Zenkteler and Zenkteler 2013). Roger Gautheret was one of the three French botanists that successfully obtained unlimited growth of plant cell cultures (Thorpe 2007). Prof. Janina Rogozińska from Agricultural Academy in Bydgoszcz also visited Gautheret’s laboratory and completed her post-doctoral in Skoog’s laboratory. Prof. Maciej Zenkteler, after his visit to Hildebrandt and Maheshwari laboratory in Delhi, developed advanced research in experimental embryology utilising in vitro tissue techniques. For many decades he conducted practical courses and training on the plant in vitro cultures for researchers from various academic centres in Poland (Zenkteler and Zenkteler 2013).
Polish botanists who became interested in employing in vitro culture of medicinal plants included Prof. Henryk Bukowiecki and Prof. Mirosława Goleniewska-Furmanowa from the Medical University of Warsaw, along with Prof. Lutosława Skrzypczak from Poznan University of Medical Sciences. Professor Mirosława Goleniewska-Furmanowa was one of the leaders in investigating the use of medicinal plants in vitro cultures to acquire important secondary metabolites. Her extensive practical experience, knowledge gained from foreign scientists, and unwavering research enthusiasm resulted in training many specialists in Poland and contributing to staff development in many botanical and pharmacognostic departments at universities of medical sciences (Zenkteler and Zenkteler 2013; Pietrosiuk et al. 2022).
Since the previous century, researchers have been developing micropropagation methods for medicinal plant production and in vitro propagation as a tool for producing beneficial secondary metabolites. The research was carried out at several other Polish academic institutions and research institutes, with newly emerging research centres also fulfilling the growing demand for medicinal plant micropropagation. As demonstrated in Table 1, the accomplishments of different Polish researchers in employing various plant micropropagation methods based on established protocols of selected taxa were reported in local and international journals. The monographs on methods for micropropagation of medicinal and aromatic plants and the analysis of their bioactive compounds, mainly secondary metabolites, have been published by Polish authors in several volumes of Springer-Verlag’s continuous series Biotechnology in Agriculture and Forestry (Furmanowa and Olszowska 1992; Furmanowa and Rapczewska 1993; Skrzypczak et al. 1993a, b, 1996, 1998, 1999). Polish journal publishing houses, especially Acta Societatis Botanicorum Poloniae (ISSN: 0001-6977), Acta Biologica Cracoviensia Series Botanica (ISSN: 0001-5296), and Herba Polonica (ISSN: 0018-0599), also play an essential role in collecting and preserving the knowledge of micropropagation protocols for medicinal plants established by Polish researchers.
At least 265 reported studies were published in English within the Polish and English publishing houses between 1980 and 2022. Figure 2 provides an overview of the development of plant biotechnology research, especially the micropropagation of herbal plants, in Poland. The number of research significantly increases in the last decade. The Polish government, national academics, and institutes paid considerable attention to and funded research and development in this area.
Journals and chapters published by Polish researchers between 1980 and 2023 on the topic of medicinal plant in vitro cultures. The number of publications were collected from three national publishing house (Acta Societatis Botanicorum Poloniae, Acta Biologica Cracoviensia Series Botanica, and Herba Polonica), and two international publishers (Springer and Elsevier)
Among academic institutes, there is Department of Pharmaceutical Botany and Plant Biotechnology at Poznan University of Medical Science (Poznań), Department of Biology and Pharmaceutical Botany at Medical University of Lodz (Łódź), Department of Pharmaceutical Biology and Medicinal Plant Biotechnology at Medical University of Warsaw (Warszawa), Department of Biology and Medicinal Plant Biotechnology at Medical College of Jagiellonian University (Cracow), Department of Biology and Pharmaceutical Botany at Wroclaw Medical University (Wrocław), Laboratory of Biologically Active Compounds at Intercollegiate Faculty of Biotechnology University of Gdansk and Medical University of Gdansk, Department of Pharmacognosy at Medical University of Gdansk (Gdańsk) and Department of Plant Physiology and Biotechnology at Nicolas Copernicus University (Torun). Polish research institutes engage in micropropagation of medicinal plants are the Department of Biotechnology at the Institute of Natural Fibers and Medicinal Plants (Poznań), Wroclaw Botanical Garden (Wrocław), Maj Institute of Pharmacology Polish Academy of Science (Cracow), and Polish Academy of Science in Cracow (Fig. 3).
Plant species in the field of interest of polish researchers
Polish researchers are pioneers in developing methods for selected medicinal plants’ micropropagation that introduces protected or foreign species to in vitro conditions for the first time. These investigations sought novel chemicals or secondary metabolite sources with biological and/or pharmacological action. The broad spectrum of plant species belonging to various genera and families studied by Polish researchers is shown in Table 1. The choice of the studied species was mainly dictated by the possibility of obtaining raw materials for phytochemical and biological studies. The conducted research was in the scope:
-
1.
Research and development of micropropagation strategies for obtaining new biotechnological raw materials as a potential source of crucial metabolites and for the search for new compounds with biological activity from well-studied or well-known species (e.g., Arnica montana, Cannabis sativa, Salvia officinalis, Chamaenerion angustifolium, Plantago spp.).
-
2.
Biomass multiplication of protected species (e.g., Drosera anglica, Eryngium maritimum, E. alpinum, Linnaea borealis, Primula veris, Rubus chamaemorus), rare taxa (e.g., E. campestre, Lychnis flos-cuculi, Drosera spp.) or endemic species (e.g., Inula verbascifolia) for phytochemical and biological studies using the in vitro technique. These taxa’s raw resources are usually unavailable because they are impossible to gather from natural sites or cannot be cultivated conventionally due to their habitat requirements.
-
3.
In vitro studies of interesting plant species from other climatic zones (e.g., Lithospermum canescens, Pueraria lobata, Rhaponticum carthamoides, Withania somnifera, Rehmannia glutinosa) and search for the new biotechnological source of desired compounds.
The developed micropropagation protocols
Micropropagation protocol development requires selecting appropriate plant material and developing methods for the multiplication of shoots and the induction and development of roots. Then further develop these whole plantlets to be ready for acclimatisation. Several parameters require optimisation throughout this process, such as: choosing the suitable media type, improving with hormonal supplementation (combination and concentration of plant growth regulators), and finding a suitable culture system. Explants applied in the multiplication process were selected after analysing the biology of the species and the availability of plant material.
Primary explants selection
Propagation of medicinal plants has been achieved in in vitro culture, usually through the proliferation of small explants such as shoot tips and axillary buds. High-quality raw materials of micropropagated plants with stable properties could be obtained via in vitro technique by collecting primary explants isolated from naturally-grown plants without causing damage to the ecosystem. Moreover, propagation from existing meristem parts yields genetically and biochemically identical multiplied plantlets to the donor plants, which is desirable and beneficial from a pharmaceutical point of view (Bajaj et al. 1988; Thiem and Kikowska 2008).
The primary explants for initiation of in vitro culture were often part of whole plants or seeds that aseptically germinated, young parts of in vitro-derived plantlets. The explants with meristematic tissue, such as shoot tips (e.g., Arnica montana, Plantago spp., Salvia spp., Pueraria lobata), axillary buds (Codonopsis pilosula, Eryngium spp., Solidago virgaurea), and nodal explants (e.g., Chamaenerion angustifolium, Thymus vulgaris), were chosen in these studies as listed in Table 1. During the subculture, shoots were multiplied via the natural development of the existing meristematic parts, unexpanded leaves at different development stages, and several primordia (Bhatia and Sharma 2015). Plantlet production in micropropagation could undergo direct organogenesis from any plant tissue or indirect organogenesis via dedifferentiated cells (Bhatia and Sharma 2015). Several studies covered in this review paper achieved plant regeneration indirectly from callus (e.g., Bergenia crassifolia, Cannabis sativa, Centaurium erythraea, Plantago media, Rhaponticum carthamoides), most often occurring on seedling or leaf explants. The direct organogenesis method was less often used to perform shoot regeneration and multiplication (e.g., Blackstonia perfoliata, Drosera spp., Rhaponticum carthamoides).
Media selection
The most widely used basic medium in the process of plant micropropagation was Murashige and Skoog (MS) and modifications thereof, such as half-strength MS (½ MS). MS basal media has the most prosperous macronutrient composition among all mentioned basic media, making good plant regeneration. However, the MS medium was not the best choice for optimum development in certain circumstances since it had a high ammonium-to-nitrate ratio (Phillips and Garda 2019). Thus, other media, such as Gamborg (B5), Nitsch and Nitsch (NN), Linsmaier and Skoog (LS), Schenk and Hildebrandt (SH), or Reinert and Mohr (RM) were used for the selected medicinal plant (Table 1).
Ammonium levels are lower in both B5 and LS media, making it the best medium for propagating Lithospermum canescens. The Nitsch and Nitsch (NN) media has approximately half of MS’s salts composition but composes of a similar ammonium-to-nitrate ratio which is ideal for shoot multiplication in Bergenia crassifolia, Coluria geoides, Thymus vulgaris, and Withania somnifera. Woody plant medium (WPM), which has lesser amounts of macronutrient salt, was used for Rhododendron tomentosum clonal propagation following its woody characteristics. In rare cases, the Fast media or Vacin and Went (VW) media were used for Drosera anglica and D. binata and Reinert and Mohr (RM) media for the propagation of Byblis liniflora (Table 1). The Fast and VW media are generally macro- and micronutrient deficient compared to others and addressed for the orchid. However, Kawiak et al. (2003) proved that these media, along with the half-strength MS, were preferred for Drosera micropropagation. Other media originally intended for orchids were also suitable for Drosera based on the protocol developed by Kukułczanka and Czastka (1988).
Studies of Drosera spp. have been aimed at determining the most favourable media conditions for their growth (Kukułczanka and Cząstka 1991; Kawiak et al. 2003). There seems to be no universal medium for all Droseraceae species. A study by Kawiak et al. (2003) identified MS medium with mineral content reduced by half as the one most effective for some Drosera species. Królicka et al. (2008) claimed that the best medium for Drosera binata propagation was Vacin and Went. Jadczak et al. (2017) indicated that the medium most favorable for the growth of D. rotundifolia was ¼ MS. The plants grown on this medium had the greatest weight and produced the most significant number of progeny plants. The number of plantlets cultured on different media—1/8 MS or ½ MS was significantly lower. The study of Jadczak (2017) also showed a clear correlation between reduced content of minerals and increased number of roots. The greatest number of long roots was developed on ¼ MS medium. Numerous roots were also developed by shoots cultures on the media with the content of nitrogen compounds reduced by half (Jadczak et al. 2017).
Plant growth regulators (PGRs)
The balance of plant growth regulators (endogenous synthesised and exogenous applied) guarantees success at every stage of micropropagation. The types and amounts of growth regulators utilised in these experiments differed between species and were chosen with the biology and physiology of a particular taxon in mind.
In light of research confirming the usefulness of PGRs, cytokinins level especially is critical for the multiplication of shoots. The 6-benzyladenine (BAP) is the first generation of synthetic cytokinins that are often added to the media individually or in combination with auxin in these studies and significantly caused the formation and development of shoots, e.g., Centaurium erythraea (44.4 S/E), Codonopsis pilosula (38.2 S/E), Linnaea borealis (17 S/E), Scutellaria alpina (36.1 S/E), and Withania somnifera (120 S/E).
Thidiazuron (TDZ) is a urea-derivative cytokinin with a dynamic role in plant tissue culture. TDZ has been applied in in vitro techniques to induce adventitious shoot formation or promote axillary shoot proliferation (Lu 1993). The action of TDZ is highly dependent upon its concentration, exposure time, and cultured explants (Ahmad and Shahzad 2018). The addition of TDZ to Cannabis sativa caused phenotypic vitrification, leaf narrowing, leaf rolling, and suppressed the growth of shoots, which are highly undesirable in biomass production (Wróbel et al. 2022). Jesionek et al. (2016) reported that a combination of TDZ and 6-(γ,γ-Dimethylallylamino)purin (2iP) on the endangered essential oil-bearing Rhododendron tomentosum efficiently produced a massive number of shoot primordia at the multiplications stage (276.3 growth index) after 30 days of cultivation. The morphology of the microshoots was highly affected by the presence of both cytokinins. The microshoots were healthy and without any signs of necrosis. However, they were short and formed calluses at the base of explants. In the study of Harpagophytum procumbens conducted by Grąbkowska et al. (2014), pretreating the explants with TDZ for 6 h and then cultured to SH medium containing 6-benzylaminopurine (BAP) and indole-3-acetic acid (IAA) increased the shoot multiplication up to 330% compared to non-pretreated explants. Nevertheless, it caused dwarfism and hyperhydricity of the microshoots. On the other hand, H. procumbens microplants pretreated with TDZ had higher survival ability and morphological features than non-pretreatment plants (Grąbkowska and Wysokińska 2009; Grąbkowska et al. 2014).
Besides cytokinin supplementation, adenine sulphate and gibberellic acid were also added to the media. Adenine sulphate (AS) acts as a precursor for natural cytokinin synthesis or enhances the natural synthesis of cytokinin. In this way, it enhances the growth and proliferation of axillary shoots and promotes adventitious shoot formation (George et al. 2008). Better shoot development was observed on media with AS in Bergenia crassifolia, Coluria geoides, Rubus chamaemorus, Thymus vulgaris, and Withania somnifera (Table 1) supplementation. Excessively high adenine sulphate concentration could additionally induce organogenesis. As a result, shoots formed from the meristematic tissue and adventitious arise via organogenesis (e.g., B. crassifolia). Gibberellic acid (GA3) is simple natural gibberellin and has been applied to culture media to promote rapid stem elongation, an essential step before rooting (Phillips and Garda 2019). Thus, it became a choice for shoot elongation by some researchers (e.g., Eryngium alpinum). The GA3 supplementation to media was the most suitable media for long-term culture and effective proliferation (Kikowska et al. 2020a, b).
Among various auxins applied for in vitro rooting, the natural auxin IAA is the most commonly used in the selected studies, such as Coluria geoides (100% R), Eustoma grandiflorum (90% R), Plantago lanceolata (100% R), and Pueraria lobata (100% R). Synthetic auxins, indole-3-butyric acid (IBA; e.g., Coluria geoides (100%R), Inula verbascifolia (100% R), and Rehmannia glutinosa (93% R), α-naphthaleneacetic acid (NAA; e.g., Drosera anglica (90%), D. cuneifolia (25%), Plantago maritima (89% R), and Salvia nemorosa (100% R)) were also used in several studies. Thiem (2003) observed the impact of NAA, IBA, and IAA supplementation in various concentrations on root induction of P. lobata. From the result, IAA promoted root induction more effectively (100% R). Nevertheless, plantlets rooted on medium with IAA grew slowly and became yellow. Meanwhile, plantlets grown on NAA had thicker and shorter roots with callus-forming capacity, which reduced the capability of plantlets to survive during acclimatisation. A combination of natural and synthetic auxin, such as IAA and NAA on Eryngium alpinum, promoted rooting up to 34.5 R/E. The supplementation of both auxins significantly improved rooting compared to in vitro rhizogenesis occurring in the case of single auxins (2.9 and 7.5 R/E on IAA and IBA, respectively). The presence of NAA in the medium induced relatively short, thick, dark roots. The generally shallow structure allows root to quickly obtain water before it evaporates. Extensive branching of those roots may extend the area of obtaining water with mineral salts, which is important in the process of planting the plant to ex vitro conditions. From a pharmaceutical point of view, the fibrous system is also more attractive because most often noticeable is higher production of desired secondary metabolites. Young roots and lateral roots are more suitable for the production of bioactive compounds (Kikowska et al. 2020a, b). Similar roots’ morphology was also observed in E. planum. The addition of IBA to the media generated lateral roots on the microplants. Meanwhile, IAA supplementation resulted in long, thin roots with fewer lateral roots. The initially whitish roots subsequently turned dark brown and decreased in length along with shoot maturation, indicating an increased secondary metabolite concentration (Thiem et al. 2013). Some plants may undergo spontaneous rooting on full-strength or half-strength media without plant growth regulators (e.g., Arnica montana, Centaurium erythraea, Lycopus lucidus, and Scutellaria alpina). Efficient root formation on auxin-free media may occur due to high endogenous auxin availability in in vitro shoots. Therefore, exogenous addition on media is not essential to induce root formation (Piątczak and Wysokińska 2003).
As previously mentioned, genetic instability may arise as the period of in vitro propagation increases and as constant exposure to a high level of plant regulators. The genetic stability assessment of in vitro propagated plants should be carried out each time while developing the protocols. This evaluation is essential to monitor and maintain the level of metabolites from the raw material. Detection based on a morphological feature only is undependable and imprecise, as phenotypically similar explants may possess different molecular properties. In order to confirm the genetic fidelity of plantlets, cytogenetic (flow cytometry) or molecular methods, such as amplified fragment length polymorphism (AFLP), inter-simple sequence repeat (ISSR), or randomly amplified polymorphic DNA (RAPD), were often applied (Sliwinska 2018).
In vitro environmental stress
Environmental stress is essential in determining plant structure and function in closed and open environments. Furthermore, it is also the key determinant for fluctuation in plant secondary metabolites (Verma and Shukla 2015). Adamczuk et al. (2012) reviewed the possible role of stress and reactive oxygen species in morphogenic process of in vitro plants. In this controled micropropagation system, osmotic stress induced by high or low sucrose content of the media, reduction in the mineral composition of the media, high concentration of exogenous plant growth regulators, high and low temperature, quality of illumination, propagation system including agitated tissue and organ cultures could trigger explants’ response to stress, varied on the species. In Codonopsis pilosula, for example, high sucrose concentration in media affected the morphology of the shoots and roots to become thicker and shorter (Słupski et al. 2011). The study of osmotic stress on the endangered Rhododendron tomentosum, presented that along the the increasing of sucrose concentration, root induction is also enhanced (Jesionek et al. 2016).
The use of the agitated culture system with total immersion was not recommended in the case of Salvia officinalis L. and Eryngium alpinum L. cultivation. Micropropagated shoots obtained by this method displayed malformations and undesirable physiological abnormalities. In these cases, it was probably an improper ratio of the volume of the medium to the culture vessel (Grzegorczyk and Wysokińska 2008; Kikowska et al. 2020a). The shoots of propagated Linnaea borealis L. immersed in higher volume of the medium in RITA bioreactor (200 ml in comparison to 150, 100 or 80 ml) were characterised by overgrowth and hyperhydricity (Kikowska et al. 2022). The stationary liquid culture for shoot multiplication of Scutellaria alpina L. was supported by polyurethane foam (PUL system) or bacterial nanocellulose (BNCL system). The concentrations of bioactive metabolites, including baicalin, verbascoside and flavones in the liquid cultures (BNCL, PUL) were significantly higher than those in shoots cultured on agar solidified medium (Grzegorczyk-Karolak et al. 2017b). The introduction of Genista species into in vitro culture conditions negatively affected the flavones production but improved the quantity of isoflavones (Łuczkiewicz and Piotrowski 2005). Their results suggested that different propagation conditions impacted the secondary metabolite production.
Propagation systems: stationary, agitated, and bioreactor
The propagation is carried on solid or liquid media stationary or agitated system, respectively (Fig. 3). The number of new shoots multiplied in liquid media was usually more significant than on solid media, as shown in the study of Drosera anglica, D. binata, Linnaea borealis, Salvia officinalis, Scutellaria alpina, Lychnis flos-cuculi (Table 1). The liquid system allows explants to have a larger surface area and faster nutrient uptake, supported by constant agitation. The application of liquid system to micropropagate D. anglica and D. binata significantly improved the proliferation rate up to twofold than in solid system (Kawiak et al. 2003). However, the fluid system is not favourable for several species due to the appearance of hyperhydricity, an unusual apoplastic water accumulation resulting in morphology abnormalities (e.g., thickened stems, short internodes, fragile and twisted leaves). Adverse effects of the liquid culture system for in vitro plants were shown in the study of D. cuneifolia (Kawiak et al. 2003) and S. alpina (Grzegorczyk-Karolak et al. 2017b). The continuous contact between plants and media might cause cultures to become necrotic and fail to proliferate. The problem was then overcome by using cellulose bridges to support the shoot cultures, allowing partial contact between plant material and liquid medium. This method improved the multiplication rate of S. alpina compared to other tested systems with very low hyperhydricity events (Grzegorczyk-Karolak et al. 2017a, b). Another solution to overcome these issues is by selecting the appropriate volume of liquid media for the size of culture vessels. As the explants are not completely immersed in the medium, vitrification of plant tissue can be avoided, as conducted by Kikowska et al. (2022) on Linnaea borealis shoot cultures.
The culture containers were mainly using Erlenmeyer flasks. Nutrient sprinkle bioreactors and temporary immersion systems were also utilised to increase the size of the culture and boost micropropagation productivity. Shoots proliferation of Rehmannia glutinosa was significantly increased when grown in a nutrient sprinkle bioreactor (NSB; 21 S/E) than in a stationary system (8.2 S/E) (Piątczak et al. 2014). The shoot cultures of L. borealis also grew efficiently in a temporary immersion system RITA® bioreactor up to 894% of fresh weight biomass ratio (Kikowska et al. 2022). However, biomass production using bioreactors does not constantly improve the yield, as observed in the study of Scutellaria alpina where the shoot multiplication rate only increased slightly compared to the solidified system (Grzegorczyk-Karolak et al. 2017b). Therefore, further optimisations of the bioreactor’s protocols for each species are highly needed.
Acclimatisation
The success of the micropropagation process depends on the plantlets’ transfer to ex vitro conditions as the final step. Multiplied whole plants are transferred into acclimatisation in order to produce plants for outplanting or reintroduction to natural habitat. Microplants transferred to ex vitro conditions are exposed to abiotic (altered temperature, light intensity, and humidity) along with biotic stress from soil microflora.
Proper rooting of the explant is crucial to ensure the plant’s survivability. In the study of Codonopsis pilosula, the author emphasised that different rooting systems obtained from in vitro propagation influenced survival rates. Roots with shorter and thicker characteristics had a higher survival rate as they could cope with soil conditions (Słupski et al. 2011). A high percentage of rooting correlates with high survival acclimatisation. Plantlets with high rooting chances had a higher survival rate, as observed in several species with (Rhaponticum carthamoides—direct organogenesis (88% R)) compared to a low rooting percentage (e.g., R. carthamoides—indirect organogenesis (59% R)) (Skała et al. 2015). The difference in humidity between in vitro (high humidity) and ex vitro (low humidity) conditions could induces abiotic stress to the plants during acclimatisation. Retardation of cuticle development, epicuticular waxes and functional stomatal apparatus could occur during in vitro culture and causes high transpiration rates of plantlets ex vitro (Chandra et al. 2010). Therefore, the authors often covered the plantlets with glass or plastic to ensure high humidity (e.g., Rehmannia glutinosa, Centaurium erythraea, and Lychnis flos-cuculi) or adjusted the light intensity by acclimatising to the dark tunnel (e.g. Chamaenerion angustifolium). Maliński et al. (2019) further observed the 98% survival rate of L. flos-cuculi from three batched of 25 plants in the experimental plot for two vegetation systems from the previous 91% survival frequency during acclimatisation in pots.
Micropropagated plants, after transfer to soil (ex vitro) conditions are often characterised by better morphology parameters (such as a higher number of branched shoots) and physiology conditions (e.g., more vigour), which then correlate with a higher biomass of the potential pharmaceutical raw materials. For example, in the second year of vegetation, the seeds of propagated Oenothera biennis and O. paradoxa plants had a higher content of oil and γ-linolenic acid than those from intact plants (Skrzypczak et al. 1994; Thiem et al. 1999). Similar results were also seen in the in vitro-derived Lychnis flos-cucuili plants, where biomass quality and quantity improved. More branched shoots with inflorescences were observed, and the sum of main ecdysteroids (20-hydroxyecdysone and polypodine B) from the flowering herb ex vitro plants was higher than the flowering herb of intact plants (Malinski et al. 2019). Kikowska et al. (2018) reported that the sum of six main saponins significantly increased in the root vigor system of in vitro-derived Eryngium planum plants grown in an experimental plot up to 7 times more naturally grown plants.
Chemical potential of in vitro propagated medicinal plants
Developing a micropropagation system is the first step in providing an alternative biomass supply for quantifying or isolating active chemicals of interest to the pharmaceutical sector. Biomass extraction from regenerated plants cultivated on a laboratory scale and in various experimental plots is part of the strategy for introducing these species into cultivation. Phenolic acids, flavonoids, phenylethanoids, triterpenoids, saponins, ecdysteroids, and essential oils were among the substances studied in phytochemical investigations of many medicinally important plant species. Several studies have revealed an increase in the production of specific secondary metabolites in the organs of micropropagated plants compared to intact plants, which Polish researchers have accomplished.
Apiaceae
The genus Eryngium (E. alpinum, E. campestre, E. maritimum, and E. planum) is part of the Saniculoideae subfamily from the family Apiaceae, and the metabolites composition was extensively studied by Kikowska et al. (2014; 2016; 2020b) and Thiem et al. (2013). Eryngium is rich in phenolic acids, flavonoids (mostly kaempferol and quercetin derivatives), triterpenoid saponins derivatives of acylated R1 and A1-barrigenols, and essential oils. These metabolites were effectively produced using in vitro culture systems and biotechnology techniques. The content of selected phenolic acids was reported to be higher in shoots and roots collected from micropropagated Eryngium species than from analogous organs of soil-grown plants. The content of the sum of phenolic acids in leaves from in vitro-derived plantlets was 1.67 higher from E. planum and 6.75 from E. maritimum than in the rosette leaves of the plants from natural sites (Thiem et al. 2013; Kikowska et al. 2014). Interesting research focuses on producing triterpenoid saponins, important compounds with promising anticancer properties, in the tissues of micropropagated E. planum plantlets. The shoots produced 8.7 times more sum of five main triterpenoid saponins than leaves of field-grown plants, and roots of micropropagated plantlets accumulated 3.3 times more than roots of field-grown plants (Kikowska et al. 2018). Essential oils obtained from E. planum rosette leaves of field-grown plants and shoots from in vitro propagated plantlets had different compositions. The in vitro multiplied shoots of E. planum are an outstanding source of (Z)-falcarinol (64.4% of essential oil), the most bioactive compounds in the falcarinol–type polyacetylenes (Thiem et al. 2011). On the other hand, the in vitro-derived shoots of E. alpinum plantlets were characterised by essential oil composed mainly of hexadecanoic acid (15.5%), spathulenol (7.5%), (E)-β-farnesene (4.9%), germacra-4(15),5,10(14)-trien-1α-ol (4.7%), and falcarinol (4.3%) (Kikowska et al. 2020b). The pronounced amounts of some sesquiterpenes in the shoot oil of E. planum, namely hydrocarbons with eremophilane and selinane skeletonm (E)-nerolidol, and two ketones β-elemenone and germacrone (Thiem et al. 2011).
Asteraceae
The Asteraceae family grows in diverse habitats and is one of the most prominent flowering plant families. The medicinal effect of members from the Asteraceae family comes from its phytochemicals, such as inulin, polyphenols, phenolic acids, flavonoids, acetylenes, sesquiterpene lactones, and triterpenes. These compounds provide antioxidant, anti-inflammatory, antimicrobial, and antiplatelet activities. Moreover, the Asteraceae family possess prebiotic, wound healing, diuretic and hepatoprotective properties (Rolnik and Olas 2021). Valuable phytochemicals, such as sesquiterpene, lactone, parthenolide, and essential oils, were successfully obtained from the in vitro biomass of Arnica montana, Inula verbacifolia, and Solidago spp., respectively (Weremczuk-Jeżyna and Wysokińska 2000; Thiem et al. 2003; Kalemba and Thiem 2004).
The essential oils obtained from four Solidago species (S. virgaurea, S. canadensis, S. gigantea, and S. graminifolia) proliferated in in vitro cultures produced the same yield and similar compositions as plants from the natural sites. The primary essential oil compound in in vitro propagated S. canadensis and S. virgaurea was α-pinene (59.5 and 25.4%, respectively), a constituent with fresh pine scent and woody flavour, and limonene (97 and 14.8%, respectively). The α-pinene plays a crucial role in the fragrance and flavour industry. α-pinene’s biological characteristics include antibacterial, antifungal, anti-leishmania, anti-inflammatory, anti-oxidative, antiapoptotic, neuroprotective, and gastroprotective effects (Allenspach and Steuer 2021). Among the studied species, germacrene D, a common sesquiterpene with insecticidal and repellent activities, was one of the major constituents in S. canadensis, S. graminifolia, and S. gigantea (15.2%, 12.1%, and 12.8%, respectively) (Kalemba and Thiem 2004). Thus, in vitro propagated plants of Solidago species could be a source of bioactive compounds, especially α-pinene, for pharmaceutical, fragrance, and flavor applications.
Stevia rebaudiana Bert. has intense sweet taste, which it owes to specific diterpene glycosides. The greatest influence on the quality of the raw material and level of sweetness are stevioside and rebaudoside A. As a herbal medicine, S. rebaudiana is a valuable source to fight microbial pathogens, good sweetener source for diabetic or hypoglycemic patients, high-blood pressure patient, and for people with obesity. The pharmaceutical industry uses glycosides to improve the taste of bitter tablet and syrups, and to produce basic components to protect teeth from caries (Doliński and Jabłonska 2015).
Droseraceae
An interesting subject of long-term interdisciplinary studies based on the collaboration of several institutions to search for new compounds with therapeutic activity is carnivorous plants from the Droseraceae family. The first research on Droseraceae was carried out in Wroclaw Botanical Garden using D. intermedia, D. rotundifolia, D. spatulata, and D. bredifolia. The laboratory of Plant Protection and Biotechnology of the Intercollegiate Faculty of Biotechnology in Gdańsk had optimised the media and in vitro culture conditions of D. anglica, D. binata, D. cuneifolia, D. capensis, D. aliciae. The in vitro culture allows the increase in the propagation rate of important compounds, such as naphthoquinones, flavonoids, antocyanins, and phenolic compounds. Banasiuk et al. 2012 had reviewed the application of in vitro culture for secondary metabolites production. The biomass of plants belonging to the Drosera genus was successfully obtained thanks to the in vitro culture technique. Biomass is the source for isolating and characterising selected naphthoquinones e.g., rossoliside (7-methylhydrojuglone 4-O-glucoside) and hydroplumbagin 4-O-glucoside from micropropagated plantlets of D. rotundifolia and D. intermedia, respectively (Budzianowski et al. 1993; 1996).
Gentianaceae
Plants from the family Gentianaceae are known for their ornamental features, yet upon the discoveries of secondary products from this family the research of these plants for their medicinal values stimulated. In Poland, the research on Gentians were boosted after the publication of the ‘Red Book’of Polish Flora (Rybczyński et al 2015). The family Gentianaceae has xanthones, secoiridoids, and flanovoids as their phytochemical characteristics (Skrzypczak et al. 1993a, b). The gentiopicroside (secoiridoid) content in Blackstonia perfoliata L. was dominant about 3.55 times higher in shoot of micropropagated plantlets compared to the ground plants (Skrzypczak et al. 1992). The presence of flavonoids in B. perfoliata in vitro shoot culture was also confirmed using the two-dimensional thin layer chromatography (2D TLC) on polyamide (Skrzypczak et al. 1996). Both gentiopicroside and flavonoids were also observed in Eustoma grandiflorum Shinn using thin layer chromatography (Skrzypczak et al. 1993a, b). Centaurium erythraea Rafn is used as herbal medicine (Centaurii herba) for its therapeutic effects as blood and kidney cleanser, indigestion treatment, healing wound and reducing sores. It is later known that secoiridoid glucosides (gentiopicroside, swertiamarin, and sweroside) are main components in these medical values of C. erythraea. The biomass of in vitro shoots of C. erythraea contained significantly higher gentiopicroside and swertiamarin, but no significant difference on sweroside content compared to wild-grown plants. Interestingly, the total secoiridoid contents were 1.9-threefold higher in shoots from micropropagated plantlets than in wild-grown plants (Piączak et al. 2005).
Lamiaceae
Lamiaceae family species are rich in essential oils, therefore, valuable in the cosmetic, flavoring, fragrance, perfumery, and pharmaceutical industries. They are often grown as culinary herbs for edible leaves. Lamiaceae’s most prominent secondary metabolites are phenols (tannins, quinones, flavonoids, lignans, and terpenoids) (Carović-Stanko et al. 2016). The essential oils of Lamiaceae were studied in the biomass of micropropagated Teucrium scorodonia (Makowczyńska et al. 2016) and Thymus vulgaris (Furmanowa and Olszowska 1992). The chemical composition of essential oils from plants and in vitro-derived was similar and characterised by high level of sesuiterpene hydrocarbons, such as β-caryophyllene, germacrene, and α-humulene. The results indicate that in vitro-derived plants maybe a potential source of essential oil with β-caryophyllene as the main component, approved as food additive. A simple procefure for rapid propagation of T. vulgaris was applied for obtaining of selected genotypes with different biological and chemical charaters, but the essential oils composition was similar and thymol, a compound with strong antimicrobial properties, was identified as the main compound (Furmanowa and Olszowska 1992). In whole shoots of Salvia officinalis micropropagated plants grown in greenhouse, the content of diterpenoid were different than in commercially available dried leaves (1.2–1.37 higher). Biotechnologically obtained biomass can be an important source of rosmarinic acid, a polyphenol constituent of many herbs for food and beverage applications, as a dietary supplement, pharmacy, and cosmetics. From their studies, the rosmarinic acid content from the whole shoots was 1.67–9.33 higher than the commercial dried leaves (Grzegorczyk and Wysokińska 2008). Extensive studies were conducted on in vitro propagation of Scutellaria alpina and its secondary metabolites production (Grzegorczyk-Karolak et al. 2015a ; 2017b). According to their findings, in vitro stationary shoots in liquid media of S. alpina are an excellent source for producing bioactive substances with diverse pharmacological properties, a flavone glucuronide (baicalin), flavonoid (wogonoside), and phenylethanoid (verbacoside), with the highest content of predominant metabolite baicalin (c.a. 18.79 mg/g) (Grzegorczyk-Karolak et al. 2015a). The scale-up of in vitro S. alpina shoot cultures using the bioreactor slightly increased the biomass production (Grzegorczyk-Karolak et al. 2017b). The essential oil composition of micropropagated shoots of Agastache rugosa appeared to be different than adult plant. The biomass of in vitro-derived shoots was rich in α-pinene, limonene, isomethone, and pulegone. While the adult plant possesses mostly estragole, a natural organic compound, which has been used as additive, flavoring agents, and fragrance, that were absent in the in vitro shoot cultures (Zielińska et al. 2011). Essential oils composition in in vitro shoot cultures was also studied in Salvia przewalskii by Skała et al. (2007). The highest essential oil constituents found in leaves of in vitro cultures were limonene and β-phellandrene, β-caryophyllene, and bornyl acetate. The micropropagated leaves consisted of higher bornyl acetate, a camphoraceous oil, (up to 3.9 times) and β-caryophyllene, a natural bicyclic sesquiterpene, (2.3 times) compared to the leaves from in vivo plants (Skała et al. 2007).
Plantaginaceae
Species from the family Plantaginaceae are also one of the most studied taxa by Polish researchers, especially from the genus Plantago. Makowczyńska and Andrzejewska-Golec described the micropropagation protocol of P. asiatica (2003), P. camtschatica (2008), and P. maritima (2009). Meanwhile, P. lanceolata and P. media were studied by Budzianowska et al. (2004, 2019). Early analysis found that iridoids are the chemotaxonomic markers of the genus Plantago (Andrzejewska-Golec et al. 1993). Further research of the Plantago species, the composition of iridoids and organ-specific phenylethanoid glucosides (e.g., acteoside and plantamajoside) were identified and measured in Plantago. Quantitative screening of the extract using TLC video densitometric showed a significant improvement in the accumulation of acteoside in shoots (93.03 mg/g dry weight) and plantamajoside in roots (44.08 mg/g) of in vitro propagated P. media. Phenylethanoids in the leaves of micropropagated plantlets, compared to soil-grown plants estimated by other authors. As the authors stated, the organ of in vitro propagated plants may be considered an alternative source of pharmacologically active phenylethanoids (Budzianowska et al. 2019). In the shoots of micropropagated P. lanceolata, acteoside and plantamajoside were 17.85 and 0.56 mg/g, respectively. P. lanceolata regenerated in vitro had a phenylethanoid composition similar to plants from natural sites, except for minor compounds (martynoside in place of isoacteoside) (Budzianowska et al. 2004).
Onagraceae
Several species of Oenothera have been known for their medicinal oils, which are assessed for cosmetic industries. A typical evening primrose in Poland, O. biennis, gains medicinal importance through the presence of γ-linoleic acid in the seeds. Skrzypczak et al. (1994) compared the oil and fatty acids content in O. biennis of seeds from in vitro-derived plants and ground cultivation. From their study, plant regeneration using the biotechnology technique slightly improved the total oil content (24%) and fatty acid contents, including γ-linoleic acid (8.2%), in comparison to intact plants (23 and 7.7%, respectively). Thiem et al. (1999) also observed that the content of γ-linoleic acid in the seeds of in vitro propagated O. ammophila and O. paradoxa after the first season of vegetation was comparable with those from intact plants. Oenothein B, a cyclic dimeric ellagitannin, is present in many medicinal plants and has been reported to have a wide range of biological activities (antioxidant, anti-inflammatory, anti-viral, antifungal, and antitumor). Chamaenerion angustifolium is one of the medicinal plants that produce oenothein B. The shoots of acclimatised C. angustifolium plantlets were shown to have 1.85 higher contents of oenothein B than the shoots of cultivated plants (Dreger et al. 2020). Dreger et al. (2022) also analysed the content of sterol in C. angustifolium in vitro plant cultures, and they concluded that micropropagated C. angustifolium cultures are a rich source of stigmasterol (375.64–577.77 mg/100 g dry weight) and campesterol (375.64–577.77 mg/100 g dry weight), which were 66.14 and 9.17 times higher, respectively, compared to the field-grown C. angustifolium plants. Both campesterol and stigmasterol have anti-inflammatory and anticarcinogenic activity.
Shoot cultures as biomass for production of important bioactive compounds
Table 2 summarizes the accomplishments of Polish scientists in utilizing shoot cultures at different developmental stages (shoot-differentiated callus, microshoots, shoot culture) within various in vitro systems (stationary, agitated, temporary immersion, liquid-phase, gas-phase). These cultures were grown on different substrates (agar-solidified, liquid) and supplemented with diverse phytohormones to serve as multiplied biomass for phytochemical research. These studies aimed to assess the ability of shoot cultures under different physicochemical conditions to biosynthesize species-specific metabolites and determine their potential as an alternative source of bioactive compounds. In order to optimize the growth of biomass and multiplication of shoots as well as the production of selected metabolites, shoot cultures were grown under variable in vitro conditions and subjected to various biotechnological treatments. These treatments included the use of elicitors, new regulators of plant growth and development (BAP derivatives), different lighting conditions (LED, monochromatic, UV lights), and cultivation in bioreactors (NSB, RITA®, and Plantform™). The qualitative and quantitative analyses focused on phenolic acids, flavonoids, phenylethanoids, lignans, iridoids, furanocoumarins, and alkaloids, revealing the ability of shoot cultures to synthesize these metabolites. Comparative studies often demonstrated higher efficiency of shoot cultures in producing the tested bioactive compounds compared to corresponding organs from natural plants, leading to the development of robust culture protocols.
Conclusion
The in vitro culture method is used to propagate medicinal plants in controlled cultivation conditions, even on a larger scale. Micropropagation techniques have been used for medicinal plants whenever vegetative reproduction was necessary to obtain homogenous plant material with phytochemical characteristics identical to the donor plant. As this method involved only organised meristems, hence it allows to obtain genetically stable and true-to-type microplants. Using in vitro technologies as dual strategy for ex situ conservation and a source of bioactive compounds of rare and protected species could be realised. The Polish researchers have already contributed to developing micropropagation protocols for these medicinally valuable plants. The pioneering studies started in the 1980s, and the legacy is still progressing until this time. Thanks to the multidisciplinary collaboration, it is possible to obtain biomass for phytochemical and biological research. Hopefully, the efforts will keep improving and lead to the successful production of biomass and valuable active compounds that benefit society.
Data availability
Data sharing is not applicable to this article as no datasets were generated.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- 2iP:
-
6-(γ,γ-Dimethylallylamino)purine
- AC:
-
Agitated culture
- AS:
-
Adenine sulfate
- B5 :
-
Gamborg medium
- BAP:
-
6-Benzylaminopurine
- DIC:
-
Dicamba
- DO:
-
Direct organogenesis
- GA3 :
-
Gibberellic acid
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- IO:
-
Indirect organogenesis
- KIN:
-
Kinetin
- LM:
-
Liquid medium
- LS:
-
Linsmayer and Skoog medium
- MS:
-
Murashige and Skoog medium
- NAA:
-
1-Naphthaleneacetic acid
- NN:
-
Nitsch and Nitsch medium
- NSB:
-
Nutrient sprinkle bioreactor
- PGRs:
-
Plant growth regulators
- R:
-
Rooting
- R/E:
-
Roots per explant
- RM:
-
Reinert and Mohr medium
- S:
-
Sucrose
- SH:
-
Schenk–Hildebrandt medium
- SR:
-
Survival rate
- SM:
-
Solid medium
- SS:
-
Stationary system
- SSM:
-
Semi-solid medium
- S/E:
-
Shoots per explant
- TDZ:
-
Thidiazuron
- TRIA:
-
Triacontanol
- VW:
-
Vacin and Went medium
- WP:
-
Woody plant medium
- ZEA:
-
Zeatin
References
Adamczuk A, Siegień I, Ciereszko I (2012) Morphogenesis of plants in vitro under stress conditions. In: Łaska G (ed) Biological diversity-from cell to ecosystem. Polish Botanical Society, Białystok, pp 25–40
Ahmad Z, Shahzad A (2018) Thidiazuron influenced morphogenesis in some medicinal plants. In: Ahmad N, Faisal M (eds) Thidiazuron: from urea derivative to plant growth regulator. Springer, Singapore, pp 231–246
Allenspach M, Steuer C (2021) α-Pinene: a never ending story. Phytochem 190:112857. https://doi.org/10.1016/j.phytochem.2021.112857
Andrzejewska-Golec E, Makowczyńska J (2008) Micropropagation of Plantago camtschatica Link. Acta Soc Bot Pol 77:269–273. https://doi.org/10.5586/asbp.2008.033
Andrzejewska-Golec E, Ofterdinger-Daegel S, Calis I, Świątek L (1993) Chemotaxonomic aspects of iridoids occurring in Plantago subg. Psyllium (Plantaginaceae). Plant Syst Evol 185:85–89
Bach A, Pawłowska B (2009) Developmental processes in in vitro culture and types of culture (in Polish). In: Malepszy S (ed) Plant biotechnology (in Polish). Wydawnictwo Naukowe PWN SA, Warsaw, pp 21–40
Bajaj YPS, Furmanowa M, Olszowska O (1988) Biotechnology of the micropropagation of medicinal and aromatic plants. In: Bajaj YPS (ed) Medicinal and aromatic plants I in agriculture and forestry. Springer, Heidelberg, pp 60–103
Balunas MJ, Kinghorn AD (2005) Drug discovery from medicinal plants. Life Sci 78:431–441. https://doi.org/10.1016/j.lfs.2005.09.012
Banasiuk R, Kawiak A, Królicka A (2012) In vitro cultures of carnivorous plants from the Drosera and Dionaea genus for the production of biologically active secondary metabolites. Biotechnologia 93(2):87–96
Bhatia S, Sharma K (2015) Micropropagation. In: Bhatia S, Sharma K, Dahiya R, Bera T (eds) Modern application of plant biotechnology in pharmaceutical science. Academic Press, Cambridge, pp 361–368
Budzianowska A, Skrzypczak L, Budzianowski J (2004) Phenylethanoid glucosides from in vitro propagated plants and callus cultures of Plantago lanceolata. Planta Med 70:834–840. https://doi.org/10.1055/s-2004-827232
Budzianowska A, Kikowska M, Małkiewicz M, Karolak I, Budzianowski J (2019) Phenylethanoid glycosides in Plantago media L. organs obtained in in vitro cultures. Acta Biol Cracov Bot 61:7–18. https://doi.org/10.24425/118060
Budzianowski J (1996) Naphthohydroquinone glucosides of Drosera rotundifolia and D. intermedia from in vitro cultures. Phytochemistry 42(4):1145–1147. https://doi.org/10.1016/0031-9422(96)00076-3
Budzianowski J, Skrzypczak L, Kukułczanka K (1993) Phenolic compounds of Drosera intermedia and D. spathulata from in vitro cultures. Acta Horticult 330(36):277–280. https://doi.org/10.17660/ActaHortic.1993.330.36
Carović-Stanko K, Petek M, Grdiša M, Pintar J, Bedeković D, Herak Ćustić M, Satovic Z (2016) Medicinal plants of the family Lamiaceae as functional foods—a review. Czech J Food Sci 34:377–390. https://doi.org/10.17221/504/2015-CJFS
Cassells AC (2012) Pathogen and biological contamination management in plant tissue culture: phytopathogens, vitro pathogens, and vitro pests. In: Ochoa-Alejo N, Loyols-Vargas V (eds) Plant Cell Culture Protocols. Methods in Molecular Biology. Humana Press, Totowa
Chandra S, Bandopadhyay R, Kumar V, Chandra R (2010) Acclimatisation of tissue culture plantlets: from laboratory to land. Biotechnol Lett 32:1199–1205. https://doi.org/10.1007/s10529-010-0290-0
Chun SC, Gopal J, Sivanesan I, Muthu M (2020) An analytical retrospection of mass spectrometric tools established for plant tissue culture: current endeavours and future perspectives. Trends Anal Chem 126:115843. https://doi.org/10.1016/j.trac.2020.115843
Debnath M, Malik CP, Bisen PS (2006) Micropropagation: a tool for the production of high quality plant-based medicines. Curr Pharm Biotechnol 7:33–49. https://doi.org/10.2174/138920106775789638
Doliński R, Jabłońska E (2015) Micropropagation of stevia (Stevia rebaudiana Bert.) through node explants isolated from in vitro produced plants (in Polish). Annal Universitatis Mariae Curie-Skłodowska. Sectio E Agricultura 70(4):13–24
Dreger M, Wegenke J, Makowiecka J, Wielgus K (2016) Application of multi-shoots cultures in micropropagation of willowherb Chamaenerion angustifolium (L.) Scop. Herba Pol 62:28–39. https://doi.org/10.1515/hepo-2016-0015
Dreger M, Gryszczyńska A, Szlata M, Wielgus K (2020) Micropropagation and HPLC-DAD, UPLC MS/MS analysis of oenothein B and phenolic acids in shoot cultures and in regenerated plants of fireweed (Chamerion angustifolium (L.) Holub). Plant Cell Tiss Organ Cult 143:653–663. https://doi.org/10.1007/s11240-020-01949-5
Dreger M, Gryszczyńska A, Szlata M, Wielgus K (2022) Content of sterols in in vitro propagated Chamerion angustifolium (L.) Holub plants. Herba Pol 68(3):1–7. https://doi.org/10.2478/hepo-2022-0016
Ekiert H, Czygan FC (2005) Accumulation of biologically active furanocoumarins in agitated cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) gams. Pharmazie 60(8):623–626
Ekiert H, Chołoniewska M, Gomółka E (2001) Accumulation of furanocoumarins in Ruta graveolens L. shoot culture. Biotechnol Lett 23:543–545
Ekiert H, Abou-Mandour AA, Czygan FC (2005) Accumulation of biologically active furanocoumarins in Ruta graveolens ssp. divaricata (Tenore) gams in vitro culture. Pharmazie 60(1):66–68
Ekiert H, Piekoszewska A, Muszyńska B, Baczyńska S (2014) Accumulation of p-coumaric acid and other bioactive phenolic acids in in vitro culture of Ruta graveolens ssp. divaricata (Tenore) gams. Medicina Internacia Revuo 102(26):24–31
Furmanowa M, Olszowska O (1992) Micropropagation of thyme (Thymus vulgaris L.). In: Bajaj YPS (ed) High-tech and micropropagation III. Biotechnology in agriculture and forestry. Springer, Heidelberg
Furmanowa M, Rapczewska L (1993) Bergenia crassifolia (L.) Fritsch (Bergenia): micropropagation and arbutin contents. In: Bajaj YPS (ed) Medicinal and aromatic plants IV. Biotechnology in agriculture and forestry. Springer, Heidelberg
Furmanowa M, Gajdzis-Kuls D, Ruszkowska J, Czarnocki Z, Obidoska G, Sadowska A, Rani R, Upadhyay SN (2001) In vitro propagation of Withania somnifera and isolation of withanolides with immunosuppressive activity. Planta Med 67:146–149. https://doi.org/10.1055/s-2001-11494
George EF, Hall MA, Klerk G-JD (2008) Plant growth regulators II: cytokinins, their analogues and antagonists. In: George EF, Hall MA, Klerk GJD (eds) Plant propagation by tissue culture. Springer, Dordrecht, pp 205–226
Grąbkowska R, Wysokińska H (2009) Micropropagation of Harpagophytum procumbens (Burch.) DC. ex Meisn.; the effect of cytokinins on shoot multiplication. Herba Pol 55(3):244–250
Grąbkowska R, Sitarek P, Wysokińska H (2014) Influence of thidiazuron (TDZ) pretreatment of shoot tips on shoot multiplication and ex vitro acclimatisation of Harpagophytum procumbens. Acta Physiol Plant 36:1661–1672. https://doi.org/10.1007/s11738-014-1541-9
Grzegorczyk I, Wysokińska H (2004) Micropropagation of Salvia officinalis L. (in Polish). Biotechnologia 2:212–218
Grzegorczyk I, Wysokińska H (2008) Liquid shoot culture of Salvia officinalis L. for micropropagation and production of antioxidant compounds; effect of triacontanol. Acta Soc Bot Pol 77:99–104. https://doi.org/10.5586/asbp.2008.013
Grzegorczyk-Karolak I, Kuźma Ł, Wysokińska H (2015a) The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpina. Plant Cell Tiss Organ Cult 122:699–708. https://doi.org/10.1007/s11240-015-0804-5
Grzegorczyk-Karolak I, Kuźma Ł, Wysokińska H (2015b) Study on the chemical composition and antioxidant activity of extracts from shoot culture and regenerated plants of Scutellaria altissima L. Acta Physiol Plant 37:1–9. https://doi.org/10.1007/s11738-014-1736-0
Grzegorczyk-Karolak I, Kuźma Ł, Wysokińska H (2016) In vitro cultures of Scutellaria alpina as a source of pharmacologically active metabolites. Acta Physiol Plant 38:1–9. https://doi.org/10.1007/s11738-015-2024-3
Grzegorczyk-Karolak I, Kuźma Ł, Wysokińska H (2017a) The influence of cytokinins on proliferation and polyphenol accumulation in shoot cultures of Scutellaria altissima L. Phytochem Lett 20:449–555. https://doi.org/10.1016/j.phytol.2016.12.029
Grzegorczyk-Karolak I, Rytczak P, Bielecki S, Wysokińska H (2017b) The influence of liquid systems for shoot multiplication, secondary metabolite production and plant regeneration of Scutellaria alpina. Plant Cell Tiss Organ Cult 128:479–486. https://doi.org/10.1007/s11240-016-1126-y
Grzegorczyk-Karolak I, Kuźma Ł, Lisiecki P, Kiss A (2019) Accumulation of phenolic compounds in different in vitro cultures of Salvia viridis L. and their antioxidant and antimicrobial potential. Phytochem Lett 30:324–332. https://doi.org/10.1016/j.phytol.2019.02.016
Grzegorczyk-Karolak I, Hnatuszko-Konka K, Zarzycka M, Kuźma Ł (2020) The stimulatory effect of purine-type cytokinins on proliferation and polyphenolic compound accumulation in shoot culture of Salvia viridis. Biomolecules 10(2):178. https://doi.org/10.3390/biom10020178
Grzegorczyk-Karolak I, Hnatuszko-Konka K, Krzemińska M, Olszewska MA, Owczarek A (2021) Cytokinin-based tissue cultures for stable medicinal plant production: regeneration and phytochemical profiling of Salvia bulleyana shoots. Biomolecules 11(10):1513. https://doi.org/10.3390/biom11101513
Grzegorczyk-Karolak I, Staniewska P, Lebelt L, Piotrowska DG (2022) Optimization of cultivation conditions of Salvia viridis L. shoots in the Plantform bioreactor to increase polyphenol production. Plant Cell Tiss Org Cult 149(1–2):269–280. https://doi.org/10.1007/s11240-021-02168-2
Grzegorczyk-Karolak I, Krzemińska M, Kiss AK, Owczarek-Januszkiewicz A, Olszewska MA (2023) Role of phytohormones in biomass and polyphenol accumulation in Salvia bulleyana in vitro culture. Biomolecules 13(2):227. https://doi.org/10.3390/biom13020227
Isah T (2015) Adjustments to in vitro culture conditions and associated anomalies in plants. Acta Biol Cracov Bot 57(2):9–28
Jadczak P, Kulpa D, Zbrojewska A (2017) In vitro micropropagation of Drosera rotundifolia. World Sci News 66:75–85
Jesionek A, Kokotkiewicz A, Włodarska P, Filipowicz N, Bogdan A, Ochocka R, Szreniawa-Sztajnert A, Zabiegala B, Bucinski A, Luczkiewicz M (2016) In vitro propagation of Rhododendron tomentosum—an endangered essential oil bearing plant from peatland. Acta Biol Cracov Bot 58(2):29–43. https://doi.org/10.1515/abcsb-2016-0019
Kalemba D, Thiem B (2004) Constituents of the essential oils of four micropropagated Solidago species. Flavour Fragr J 19:40–43. https://doi.org/10.1002/ffj.1271
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3(13):1222–1239. https://doi.org/10.5897/JMPR.9000026
Kawiak A, Królicka A, Lojkowska E (2003) Direct regeneration of Drosera from leaf explants and shoot tips. Plant Cell Tiss Organ Cult 75:175–178. https://doi.org/10.1023/A:1025023800304
Kawiak A, Królicka A, Łojkowska E (2011) In vitro cultures of Drosera aliciae as a source of a cytotoxic naphthoquinone: ramentaceone. Biotechnol Let 33:2309–2316. https://doi.org/10.1007/s10529-011-0700-y
Kawka B, Kwiecień I, Ekiert H (2017) Influence of culture medium composition and light conditions on the accumulation of bioactive compounds in shoot cultures of Scutellaria lateriflora L. (American Skullcap) grown in vitro. Appl Biochem Biotechnol 183:1414–1425. https://doi.org/10.1007/s12010-017-2508-2
Kikowska M, Thiem B, Sliwinska E, Rewers M, Kowalczyk M, Stochmal A, Oleszek W (2014) The effect of nutritional factors and plant growth regulators on micropropagation and production of phenolic acids and saponins from plantlets and adventitious root cultures of Eryngium maritimum L. Plant Growth Regul 33:809–819. https://doi.org/10.1007/s00344-014-9428-y
Kikowska M, Thiem B, Sliwinska E, Rewers M, Kowalczyk M, Stochmal A, Dlugaszewska J (2016) Micropropagation of Eryngium campestre L. via shoot culture provides valuable uniform plant material with enhanced content of phenolic acids and antimicrobial activity. Acta Biol Cracov Bot 58(1):43–56. https://doi.org/10.1515/abcsb-2016-0009
Kikowska M, Kowalczyk M, Stochmal A, Thiem B (2018) Enhance accumulation of triterpenoid saponins in in vitro plantlets and dedifferentiated cultures of Eryngium planum L.: a medicinal plant. Hort Env Biotechnol 60:147–154. https://doi.org/10.1007/s13580-018-0103-2
Kikowska M, Włodarczyk A, Rewers M, Sliwinska E, Studzińska-Sroka E, Witkowska-Banaszczak E, Stochmal A, Żuchowski J, Dlugaszewska J, Thiem B (2019) Micropropagation of Chaenomeles japonica: a step towards production of polyphenol-rich extracts showing antioxidant and antimicrobial activities. Molecules 24:1–15. https://doi.org/10.3390/molecules24071314
Kikowska M, Kalemba D, Dlugaszewska J, Thiem B (2020a) Chemical composition of essential oils from rare and endangered species—Eryngium maritimum L. and E. alpinum L. Plants 9(4):417. https://doi.org/10.3390/plants9040417
Kikowska M, Sliwinska E, Thiem B (2020b) Micropropagation and production of somatic seeds for short-term storage of the endangered species Eryngium alpinum L. Plants 9:498. https://doi.org/10.3390/plants9040498
Kikowska M, Danek K, Gornowicz-Porowska J, Thiem B (2022) Application of temporary immersion system RITA® for efficient biomass multiplication and production of artificial seeds for ex situ conservation of Linnaea borealis L. Plant Cell Tiss Organ Cult 151:673–680. https://doi.org/10.1007/s11240-022-02381-7
Klimek-Szczykutowicz M, Szopa A, Blicharska E, Dziurka M, Komsta Ł, Ekiert H (2019) Bioaccumulation of selected macro-and microelements and their impact on antioxidant properties and accumulation of glucosinolates and phenolic acids in in vitro cultures of Nasturtium officinale (watercress) microshoots. Food Chem 300:125184. https://doi.org/10.1016/j.foodchem.2019.125184
Klimek-Szczykutowicz M, Dziurka M, Blažević I, Đulović A, Granica S, Korona-Glowniak I, Ekiert H, Szopa A (2020a) Phytochemical and biological activity studies on Nasturtium officinale (watercress) microshoot cultures grown in RITA® temporary immersion systems. Molecules 25(22):5257. https://doi.org/10.3390/molecules25225257
Klimek-Szczykutowicz M, Szopa A, Dziurka M, Komsta Ł, Tomczyk M, Ekiert H (2020b) The influence of Nasturtium officinale R. Br. agar and agitated microshoot culture media on glucosinolate and phenolic acid production, and antioxidant activity. Biomolecule 10(9):1216. https://doi.org/10.3390/biom10091216
Klimek-Szczykutowicz M, Dziurka M, Blažević I, Đulović A, Miazga-Karska M, Klimek K, Ekiert H, Szopa A (2021) Precursor-boosted production of metabolites in Nasturtium officinale microshoots grown in plantform bioreactors, and antioxidant and antimicrobial activities of biomass extracts. Molecules 26(15):4660. https://doi.org/10.3390/molecules26154660
Klimek-Szczykutowicz M, Dziurka M, Blažević I, Đulović A, Apola A, Ekiert H, Szopa A (2022) Impacts of elicitors on metabolite production and on antioxidant potential and tyrosinase inhibition in watercress microshoot cultures. Appl Microbiol Biotechnol 106:619–633. https://doi.org/10.1007/s00253-021-11743-8
Kokotkiewicz A, Luczkiewicz M, Hering A, Ochocka R, Gorynski K, Bucinski A, Sowinski P (2012) Micropropagation of Cyclopia genisoides, an endemic South African plant of economic importance. Z Naturforsch 67:65–76
Królicka A, Szpitter A, Gilgenast E, Romanik G, Kaminski M, Lojkowska E (2008) Stimulation of antibacterial naphthoquinones and flavonoids accumulation in carnivorous plants grown in vitro by addition of elicitors. Enzyme Microb Technol 42(3):216–221. https://doi.org/10.1016/j.enzmictec.2007.09.011
Kukułczanka K, Cząstka B (1991) Vegetative propagation of selected species of Droseraceae taxa and in vitro gene bank establishment (in Polish). Prac Ogr Bot PAN 1: 55–61
Kukułczanka K, Czastka B (1988) In vitro culture of Drosera sp. Acta horticulturae 226: international society for horticultural science. Springer, Leuven, pp 631–634
Kuźma Ł, Kalemba D, Różalski M, Różalska B, Więckowska-Szakiel M, Krajewska U, Wysokińska H (2009) Chemical composition and biological activities of essential oil from Salvia sclarea plants regenerated in vitro. Molecules 14(4):1438–1447. https://doi.org/10.3390/molecules14041438
Kwiecień I, Smolin J, Beerhues L, Ekiert H (2018) The impact of media composition on production of flavonoids in agitated shoot cultures of the three Hypericum perforatum L. cultivars ‘Elixir’,‘Helos’,and ‘Topas.’ In Vitro Cell Dev Biol Plant 54:332–340. https://doi.org/10.1007/s11627-018-9900-7
Kwiecień I, Miceli N, D’Arrigo M, Marino A, Ekiert H (2022) Antioxidant potential and enhancement of bioactive metabolite production in in vitro cultures of Scutellaria lateriflora L. by biotechnological methods. Molecules 27(3):1140. https://doi.org/10.3390/molecules27031140
Kwiecień I, Łukaszyk A, Miceli N, Taviano MF, Davì F, Kędzia E, Ekiert H (2023a) In vitro cultures of Scutellaria brevibracteata subsp. subvelutina as a source of bioactive phenolic metabolites. Molecules 28(4):1785. https://doi.org/10.3390/molecules28041785
Kwiecień I, Miceli N, Kędzia E, Cavò E, Taviano MF, Beerhues L, Ekiert H (2023b) Different types of Hypericum perforatum cvs. (Elixir, Helos, Topas) in vitro cultures: a rich source of bioactive metabolites and biological activities of biomass extracts. Molecules 28(5):2376. https://doi.org/10.3390/molecules28052376
Lu C-Y (1993) The use of thidiazuron in tissue culture. In Vitro Cell Dev Biol Plant 29:92–96. https://doi.org/10.1007/BF02632259
Łuczkiewicz M, Piotrowski A (2005) Two-stage system for micropropagation of several Genista plants producing large amounts of phytoestrogens. Z Naturforsch C 60(7–8):557–566. https://doi.org/10.1515/znc-2005-7-808
Makowczyńska J, Andrzejewska-Golec E (2003) Micropropagation of Plantago asiatica L. through culture of shoot-tips. Acta Soc Bot Pol 72:191–194. https://doi.org/10.5586/asbp.2003.025
Makowski W, Królicka A, Tokarz B, Szopa A, Ekiert H, Tokarz KM (2023) Temporary immersion bioreactors as a useful tool for obtaining high productivity of phenolic compounds with strong antioxidant properties from Pontechium maculatum. Plant Cell Tiss Organ Cult 153(3):525–537. https://doi.org/10.1007/s11240-023-02487-6
Makowczyńska J, Andrzejewska-Golec E (2009) Micropropagation of Plantago maritima L.—a vanishing species in Poland. Acta Soc Bot Pol 78:13–18. https://doi.org/10.5586/asbp.2009.002
Makowczyńska J, Sliwinska E, Kalemba D, Piątczak E, Wysokińska H (2016) In vitro propagation, DNA content and essential oil composition of Teucrium scorodonia L. ssp. scorodonia. Plant Cell Tiss Organ Cult 127:1–13. https://doi.org/10.1007/s11240-016-1024-3
Makowski W, Tokarz B, Banasiuk R, Królicka A, Dziurka M, Wojciechowska R, Tokarz KM (2019) Is a blue–red light a good elicitor of phenolic compounds in the family Droseraceae? A comparative study. J Photochem Photobiol B 201:111679. https://doi.org/10.1016/j.jphotobiol.2019.111679
Makowski W, Tokarz KM, Tokarz B, Banasiuk R, Witek K, Królicka A (2020) Elicitation-based method for increasing the production of antioxidant and bactericidal phenolic compounds in Dionaea muscipula. J Ellis Tissue Mol 25(8):1794. https://doi.org/10.3390/molecules25081794
Maliński MP, Kikowska M, Kruszka D, Napierała M, Florek E, Sliwinska E, Thiem B (2019) Various in vitro systems of ragged robin (Lychnis flos-cuculi L.): a new potential source of phytoecdysteroids? Plant Cell Tiss Organ Cult 139:39–52. https://doi.org/10.1007/s11240-019-01660-0
Matkowski A (2008) Plant in vitro culture for the production of antioxidants—a review. Biotechnol Adv 26:548–560. https://doi.org/10.1016/j.biotechadv.2008.07.001
Moraes RM, Cerdeira AL, Lourenço MV (2021) Using micropropagation to develop medicinal plants into crops. Molecules. https://doi.org/10.3390/molecules26061752
Morozowska M, Wesołowska M (2004) In vitro propagation of Primula veris L. and preliminary phytochemical analysis. Acta Biol Cracov Bot 46:169–175
Murch SJ, Peiris SE, Liu CZ, Saxena PK (2004) In vitro conservation and propagation of medicinal plants. Biodiversity 5(2):19–24. https://doi.org/10.1080/14888386.2004.9712725
Muszyńska B, Ekiert H, Kwiecień I, Maślanka A, Zodi R, Beerhues L (2014) Comparative analysis of therapeutically important indole compounds in in vitro cultures of Hypericum perforatum cultivars by HPLC and TLC analysis coupled with densitometric detection. Nat Prod Commun 9(10):1
Ngo LT, Okogun JI, Folk WR (2013) 21st century natural product research and drug development and traditional medicines. Nat Prod Rep 30(4):584–592. https://doi.org/10.1039/C3NP20120A
Olszowska O, Furmanowa M (1993) Coluria geoides Ledeb.: micropropagation, root culture, and the production of eugenol. In: Bajaj YPS (ed) Medicinal and aromatic plants. Springer, Berlin Heidelberg, Heidelberg, pp 132–147
Pence VC (2010) Evaluating costs for the in vitro propagation and preservation of endangered plants. In Vitro Cell Dev Biol Plant 47:176–187. https://doi.org/10.1007/s11627-010-9323-6
Phillips GC, Garda M (2019) Plant tissue culture media and practices: an overview. In Vitro Cell Dev Biol Plant 55:242–257. https://doi.org/10.1007/s11627-019-09983-5
Piatczak E, Wielanek M, Wysokinska H (2005) Liquid culture system for shoot multiplication and secoiridoid production in micropropagated plants of Centaurium erythraea Rafn. Plant Sci 168(2):431–437. https://doi.org/10.1016/j.plantsci.2004.08.013
Piątczak E, Wysokińska H (2003) In vitro regeneration of Centaurium erythraea Rafn from shoot tips and other seedling explants. Acta Soc Bot Pol 72:283–288. https://doi.org/10.5586/asbp.2003.036
Piątczak E, Grzegorczyk-Karolak I, Wysokińska H (2014) Micropropagation of Rehmannia glutinosa Libosch.: production of phenolics and flavonoids and evaluation of antioxidant activity. Acta Physiol Plant 36:1693–1702. https://doi.org/10.1007/s11738-014-1544-6
Piątczak E, Kuźma Ł, Kozłowska W, Lisiecki P, Szemraj M, Płachno BJ, Gonciarz W, Chmiela M, Matkowski A, Zielińska S (2020) Phenylethanoid and iridoid glycosides production in Rehmannia elata NE Brown ex Prein. in vitro shoot cultures and their biological activity. Ind Crops Prod 158:113050. https://doi.org/10.1016/j.indcrop.2020.113050
Pietrosiuk A, Budzianowska A, Budzianowski J, Ekiert H, Jeziorek M, Kawiak A, Kikowska M, Krauze-Baranowska M, Królicka A, Kuźma Ł, Łuczkiewicz M, Malarz J, Matkowski A, Stojakowska A, Sykłowska-Baranek K, Szopa A, Szypuła W, Thiem B, Zielińska S (2022) Polish achievements in bioactive compound production from in vitro plant culture. Acta Soc Bot Pol 91:9110. https://doi.org/10.5586/asbp.9110
Płażek A, Dubert F (2022) In vitro culture as a tool for studying plant developmental processes at the physiological level in Poland. Acta Soc Bot Pol 91:9113. https://doi.org/10.5586/asbp.9113
Piątczak E, Królicka A, Wysokińska H (2011) Morphology secoiridoid content and RAPD analysis of plants regenerated from callus of Centaurium erythraea Rafn. Acta Biol Cracov Bot 53(2):79–86. https://doi.org/10.2478/v10182-011-0030-3
Podwyszyńska M, Orlikowska T, Trojak-Goluch A, Wojtania A (2022) Application and improvement of in vitro culture systems for commercial production of ornamental, fruit, and industrial plants in Poland. Acta Soc Bot Pol 91:914. https://doi.org/10.5586/asbp.914
Ptak A, Simlat M, Kwiecień M, Laurain-Mattar D (2012) Leucojum aestivum plants propagated in in vitro bioreactor culture and on solid media containing cytokinins. Eng Life Sci 13(3):261–270. https://doi.org/10.1002/elsc.201200109
Purohit SD, Teixeira da Silva J, Habibi N (2011) Current approaches for cheaper and better micropropagation technologies. Int J Plant Dev Biol 5(1):1–36
Rolnik A, Olas B (2021) The plants of the Asteraceae family as agents in the protection of human health. Int J Mol Sci 22:1–10. https://doi.org/10.3390/ijms22063009
Rout GR, Samantaray S, Das P (2000) In vitro manipulation and propagation of medicinal plants. Biotechnol Adv 18:91–120. https://doi.org/10.1016/s0734-9750(99)00026-9
Rybczyński J, Mikuła A (2006) Engagement of biotechnology in the protection of threatened plant species in Poland. Biodiv Res Conserv 3–4:361–368
Rybczyński J, Davey M, Mikuła A (2015) The Gentianaceae: biotechnology and applications. Springer, Heidelberg
Schlauer J, Budzianowski J, Kukułczanka K, Ratajczak L (2004) Acteoside and related phenylethanoid glycosides in Byblis liniflora Salisb. plants propagated in vitro and its systematic significance. Acta Soc Bot Pol 73(1):9–15. https://doi.org/10.5586/asbp.2004.002
Skała E, Wysokińska H (2001) Micropropagation of Salvia sclarea L. and Salvia splendens Ker. Gawlc (in Polish). Ann Univ Mariae Curie-Skłodowska Lublin 9:319–325
Skała E, Wysokińska H (2004) In vitro regeneration of Salvia nemorosa L. from shoot tips and leaf explants. In Vitro Cell Dev Biol Plant 40:596–602. https://doi.org/10.1079/IVP2004580
Skała E, Kalemba D, Wajs A, Różalski M, Krajewska U, Różalska B, Więckowska-Szakiel M, Wysokinska H (2007) In vitro propagation and chemical and biological studies of the essential oil of Salvia przewalskii Maxim. Z Naturforsch C 62:839–848
Skała E, Grąbkowska R, Sitarek P, Kuźma Ł, Błauż A, Wysokińska H (2015) Rhaponticum carthamoides regeneration through direct and indirect organogenesis, molecular profiles and secondary metabolite production. Plant Cell Tiss Organ Cult 123:83–98. https://doi.org/10.1007/s11240-015-0816-1
Skrzypczak L, Wesołowska M, Krupińska A, Thiem B (1992) In vitro cultures of Blackstonia perfoliata (L.) Hudson and an assay of the secoiridoid content. Acta Soc Bot Pol 61:359–368. https://doi.org/10.5586/asbp.1992.031
Skrzypczak L, Wesołowska M, Budzianowski J (1993a) Eustoma grandiflorum Shinn (Texas Bluebell): callus culture, micropropagation, and the production of gentiopicroside and other secondary metabolites. In: Bajaj YPS (ed) Medicinal and aromatic plants X. Springer, Heidelberg, pp 192–201
Skrzypczak L, Wesołowska M, Skrzypczak E (1993b) Gentiana Species: in vitro culture, regeneration, and production of secoiridoid glucosides. In: Bajaj YPS (ed) Medicinal and aromatic plants IV. Springer, Heidelberg, pp 172–186
Skrzypczak L, Skrzypczak-Pietraszek E, Lamer-Zarawska E, Hojden B (1994) Micropropagation of Oenothera biennis L. and an assay of fatty acids. Acta Soc Bot Pol 63:173–177. https://doi.org/10.5586/asbp.1994.023
Skrzypczak L, Wesołowska M, Thiem B, Budzianowski J (1996) Blackstonia perfoliata (L.) Hudson (Yellow Wort): in vitro culture and the production of gentiopicroside and other secondary metabolites. In: Bajaj YPS (ed) Medicinal and aromatic plants IX. Springer, Heidelberg, pp 64–75
Skrzypczak L, Thiem B, Wesołowska M (1998) Oenothera species (evening primrose). In vitro regeneration, production of flavonoids, fatty acids, and other secondary metabolites. In: Bajaj YPS (ed) Medicinal and aromatic plants X. Springer, Heidelberg, pp 286–304
Skrzypczak L, Wesołowska M, Thiem B, Budzianowski J (1999) Solidago L. species (Goldenrod): in vitro regeneration and biologically active secondary metabolites. In: Bajaj YPS (ed) Medicinal and aromatic plants XI. Springer, Heidelberg, pp 384–403
Skrzypczak-Pietraszek E, Słota J, Pietraszek J (2014) The influence of L-phenylalanine, methyl jasmonate and sucrose concentration on the accumulation of phenolic acids in Exacum affine Balf. f. ex Regel shoot culture. Acta Biochim Pol. https://doi.org/10.18388/abp.2014_1922
Skrzypczak-Pietraszek E, Piska K, Pietraszek J (2018a) Enhanced production of the pharmaceutically important polyphenolic compounds in Vitex agnus castus L. shoot cultures by precursor feeding strategy. Eng Life Sci 18(5):287–297. https://doi.org/10.1002/elsc.201800003
Skrzypczak-Pietraszek E, Reiss K, Żmudzki P, Pietraszek J (2018b) Enhanced accumulation of harpagide and 8-O-acetyl-harpagide in Melittis melissophyllum L. agitated shoot cultures analyzed by UPLC-MS/MS. PLoS ONE 13(8):e0202556. https://doi.org/10.1371/journal.pone.0202556
Skrzypczak-Pietraszek E, Urbańska A, Żmudzki P, Pietraszek J (2019) Elicitation with methyl jasmonate combined with cultivation in the Plantform™ temporary immersion bioreactor highly increases the accumulation of selected centellosides and phenolics in Centella asiatica (L.) Urban shoot culture. Eng Life Sci 19(12):931–943. https://doi.org/10.1002/elsc.201900051
Sliwinska E (2018) Flow cytometry—a modern method for exploring genome size and nuclear DNA synthesis in horticultural and medicinal plant species. Folia Hort 30(1):103–128
Sliwinska E, Thiem B (2007) Genome size stability in six medicinal plant species propagated in vitro. Biol Plant 51:556–558. https://doi.org/10.1007/s10535-007-0121-x
Śliwińska A, Naliwajski MR, Pietrosiuk A, Sykłowska-Baranek K (2021) In vitro response of Polyscias filicifolia (Araliaceae) shoots to elicitation with alarmone–diadenosine triphosphate, methyl jasmonate, and salicylic acid. Cells 10(2):419. https://doi.org/10.3390/cells10020419
Słupski W, Tubek B, Matkowski A (2011) Micropropagation of Codonopsis pilosula (Franch.) Nannf by axillary shoot multiplication. Acta Biol Cracov Bot 53(2):87–83. https://doi.org/10.2478/v10182-011-0031-2
Ślusarkiewicz-Jarzina A, Ponitka A, Kaczmarek Z (2005) Influence of cultivar, explant source and plant growth regulator on callus induction and plant regeneration of Cannabis sativa L. Acta Biol Cracov Bot 47:145–151
Smetanska I (2008) Production of secondary metabolites using plant cell cultures. Adv Biochem Eng Biotechnol 111:187–228. https://doi.org/10.1007/10_2008_103
Stojakowska A (1992) Micropropagataion of Urginea maritima (L.) Baker S. Str Acta Soc Bot Pol 62:1–2
Stojakowska A, Malarz J (2004) In vitro propagation of Inula royleana DC. Acta Soc Bot Pol 73(1):5–8
Stojakowska A, Malarz J, Kohmünzer S (1999) Micropropagation of Scutellaria baicalensis Georgi. Acta Soc Bot Pol 68(2):103–107
Sucholas J, Ukhanova M, Greinwald A, Luick R (2021) Wild collection of medicinal and aromatic plants (MAPs) for commercial purposes in Poland—a system’s analysis. Herba Pol 67(3):1–18
Sykłowska-Baranek K, Pietrosiuk I, Dłuska H, Furmanowa M (2004) Clonal multiplication of Lithospermum canescens [Michx.] Lehm. and Onosma paniculatum [Bur. and Franch]. Herba Pol 50(2):55–64
Szewczyk A, Marino A, Molinari J, Ekiert H, Miceli N (2022) Phytochemical characterization, and antioxidant and antimicrobial properties of agitated cultures of three rue species: Ruta chalepensis, Ruta corsica, and Ruta graveolens. Antioxidants 11(3):592. https://doi.org/10.3390/antiox11030592
Szopa A, Ekiert H (2011) Lignans in Schisandra chinensis in vitro cultures. Pharmazie 66(8):633–634
Szopa A, Ekiert H (2012) In vitro cultures of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine)—a potential biotechnological rich source of therapeutically important phenolic acids. Appl Biochem Biotechnol 166:1941–1948. https://doi.org/10.1007/s12010-012-9622-y
Szopa A, Ekiert H (2014) Production of biologically active phenolic acids in Aronia melanocarpa (Michx.) Elliott in vitro cultures cultivated on different variants of the Murashige and Skoog medium. Plant Growth Regul 72:51–58. https://doi.org/10.1007/s10725-013-9835-2
Szopa A, Ekiert H (2016) The importance of applied light quality on the production of lignans and phenolic acids in Schisandra chinensis (Turcz.) Baill. cultures in vitro. Plant Cell Tiss Org Cult 127:115–121. https://doi.org/10.1007/s11240-016-1034-1
Szopa A, Ekiert H, Szewczyk A, Fugas E (2012) Production of bioactive phenolic acids and furanocoumarins in in vitro cultures of Ruta graveolens L. and Ruta graveolens ssp. divaricata (Tenore) Gams. under different light conditions. Plant Cell Tiss Org Cult 110:329–336. https://doi.org/10.1007/s11240-012-0154-5
Szopa A, Kokotkiewicz A, Marzec-Wróblewska U, Bucinski A, Luczkiewicz M, Ekiert H (2016) Accumulation of dibenzocyclooctadiene lignans in agar cultures and in stationary and agitated liquid cultures of Schisandra chinensis (Turcz.) Baill. Appl Microbiol Biotechnol 100:3965–3977. https://doi.org/10.1007/s00253-015-7230-9
Szopa A, Kokotkiewicz A, Luczkiewicz M, Ekiert H (2017) Schisandra lignans production regulated by different bioreactor type. J Biotechnol 247:11–17. https://doi.org/10.1016/j.jbiotec.2017.02.007
Szopa A, Kokotkiewicz A, Król A, Luczkiewicz M, Ekiert H (2018a) Improved production of dibenzocyclooctadiene lignans in the elicited microshoot cultures of Schisandra chinensis (Chinese magnolia vine). Appl Microbiol Biotechnol 102:945–959. https://doi.org/10.1007/s00253-017-8640-7
Szopa A, Starzec A, Ekiert H (2018b) The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia × prunifolia. J Photochem Photobiol B 179:91–97. https://doi.org/10.1016/j.jphotobiol.201
Szopa A, Klimek-Szczykutowicz M, Kokotkiewicz A, Dziurka M, Luczkiewicz M, Ekiert H (2019) Phenolic acid and flavonoid production in agar, agitated and bioreactor-grown microshoot cultures of Schisandra chinensis cv. Sadova No. 1–a valuable medicinal plant. J Biotechnol 305:61–70. https://doi.org/10.1016/j.jbiotec.2019.08.021
Szopa A, Dziurka M, Kubica P, Jafernik K, Siomak O, Ekiert H (2022) Stimulation of Lignan production in Schisandra rubriflora in vitro cultures by elicitation. Molecules 27(19):6681. https://doi.org/10.3390/molecules27196681
Taraszkiewicz A, Jafra S, Skrzypczak A, Kaminski M, Królicka A (2012) Antibacterial activity of secondary metabolites from in vitro culture of Drosera gigantea against the plant pathogenic bacteria Pseudomonas syringae pv. syringae and P. syringae pv. morsprunorum. J Plant Pathol 94(1):63–68
Tasheva K, Kosturkova G (2013) Role of biotechnology for protection of endangered medicinal plants. In: Petre M (ed) Environmental biotechnology—new approaches and prospective applications. Intech Open, Rijeka, pp 235–285
Thiem B (2001) Micropropagation of cloudberry (Rubus chamaemorus L.) by initiation of axillary shoots. Acta Soc Bot Pol 70:11–16. https://doi.org/10.5586/asbp.2001.002
Thiem B (2003) In vitro propagation of isoflavone-producing Pueraria lobata (Willd.) Ohwi. Plant Sci 165:1123–1128. https://doi.org/10.1016/S0168-9452(03)00320-0
Thiem B, Kikowska M (2008) The assurance of medicinal plants quality propagated in in vitro cultures (in Polish). Herba Pol 54:168–178
Thiem B, Skrzypczak L, Lamer-Zarawska E (1999) Micropropagation of selected Oenothera species and preliminary studies on their secondary metabolites. Acta Soc Bot Pol 68:15–20. https://doi.org/10.5586/asbp.1999.003
Thiem B, Wesołowska M, Cis J (2003) In vitro culture of Inula verbascifolia ssp. aschersoniana and production of parthenolide. Herba Pol 49:37–43
Thiem B, Budzianowski J, Wesołowska M, Ratajczak L, Morozowska M, Skrzypczak L (2008) Secondary metabolites of in vitro cultures of selected Polish rare and endangered plants. Herba Pol 54:158–167
Thiem B, Kikowska M, Kurowska A, Kalemba D (2011) Essential oil composition of different parts and in vitro shoot culture of Eryngium planum L. Molecules 16:7115–7124. https://doi.org/10.3390/molecules16087115
Thiem B, Kikowska M, Krawczyk A, Więckowska B, Sliwinska E (2013) Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tiss Organ Cult 114:197–206. https://doi.org/10.1007/s11240-013-0315-1
Thiem B, Kruszka D, Turowska N, Sliwinska E, Berge V, Kikowska M (2021) Linnaea borealis L. var. borealis—in vitro cultures and phytochemical screening as a dual strategy for its ex situ conservation and a source of bioactive compounds of the rare species. Molecules 26(22):6823. https://doi.org/10.3390/molecules26226823
Thorpe TA (2007) History of plant tissue culture. Mol Biotechnol 37:169–180. https://doi.org/10.1007/s12033-007-0031-3
Tyagi S, Rajpurohit D, Sharma A (2021) Genetic fidelity studies for testing true-to-type plants in some horticultural and medicinal crops using molecular markers. In: Kumar Srivastava D, Kumar Thakur A, Kumar P (eds) Agricultural biotechnology latest research and trends. Springer, Singapore
van der Linde PCG (2000) Certified plant from tissue culture. Acta Hortic 530:93–102. https://doi.org/10.17660/ActaHortic.2000.530.9
Verma N, Shukla S (2015) Impact of various factors responsible for fluctuation in plant secondary metabolites. J Appl Res Med Aromat Plants 2(4):105–113. https://doi.org/10.1016/j.jarmap.2015.09.002
Wawrosch C, Zotchev SB (2021) Production of bioactive plant secondary metabolites through in vitro technologies—status and outlook. Appl Microbiol Biotechnol 105:6649–6668. https://doi.org/10.1007/s00253-021-11539-w
Weremczuk-Jeżyna I, Wysokińska H (2000) Micropropagation of Arnica montana L. and sesquiterpene lactones in regenerated plants (in Polish). Biotechnologia 4:118–122
Weremczuk-Jeżyna I, Kuźma Ł, Kiss AK, Grzegorczyk-Karolak I (2018) Effect of cytokinins on shoots proliferation and rosmarinic and salvianolic acid B production in shoot culture of Dracocephalum forrestii WW Smith. Acta Physiol Plant. https://doi.org/10.1007/s11738-018-2763-z
Weremczuk-Jeżyna I, Kochan E, Szymczyk P, Lisiecki P, Kuźma Ł, Grzegorczyk-Karolak I (2019a) The antioxidant and antimicrobial properties of phenol-rich extracts of Dracocephalum forrestii WW Smith shoot cultures grown in the nutrient sprinkle bioreactor. Phytochemistry Lett 30:254–260. https://doi.org/10.1016/j.phytol.2019.01.032
Weremczuk-Jeżyna I, Skała E, Kuźma Ł, Kiss AK, Grzegorczyk-Karolak I (2019b) The effect of purine-type cytokinin on the proliferation and production of phenolic compounds in transformed shoots of Dracocephalum forrestii. J Biotechnol 306:125–133. https://doi.org/10.1016/j.jbiotec.2019.09.014
Weremczuk-Jeżyna I, Lisiecki P, Gonciarz W, Kuźma Ł, Szemraj M, Chmiela M, Grzegorczyk-Karolak I (2020) Transformed shoots of Dracocephalum forrestii WW Smith from different bioreactor systems as a rich source of natural phenolic compounds. Molecules 25(19):4533. https://doi.org/10.3390/molecules25194533
Weremczuk-Jeżyna I, Hnatuszko-Konka K, Lebelt L, Grzegorczyk-Karolak I (2021a) The protective function and modification of secondary metabolite accumulation in response to light stress in Dracocephalum forrestii shoots. Int J Mol Sci 22(15):7965. https://doi.org/10.3390/ijms22157965
Weremczuk-Jeżyna I, Kuźma Ł, Grzegorczyk-Karolak I (2021b) The effect of different light treatments on morphogenesis, phenolic compound accumulation and antioxidant potential of Dracocephalum forrestii transformed shoots cultured in vitro. J Photochem Photobiol B 224:112329. https://doi.org/10.1016/j.jphotobiol.2021.112329
Weremczuk-Jeżyna I, Lebelt L, Piotrowska DG, Gonciarz W, Chmiela M, Grzegorczyk-Karolak I (2023) The optimization growth of Dracocephalum forrestii in RITA® bioreactor, and preliminary screening of the biological activity of the polyphenol rich extract. Acta Sci Pol Hortorum Cultus Hort 22(2):45–59. https://doi.org/10.24326/asphc.2023.4817
Wróbel T, Dreger M, Wielgus K, Słomski R (2022) Modified nodal cuttings and shoot tips protocol for rapid regeneration of Cannabis sativa L. J Nat Fibers 19:536–545. https://doi.org/10.1080/15440478.2020.1748160
Zenkteler M, Zenkteler E (2013) 65 years of in vitro culture in Poland. Acta Soc Bot Pol 82(3):183–192. https://doi.org/10.5586/asbp.2013.022
Zielińska S, Piątczak E, Kalemba D, Matkowski A (2011) Influence of plant growth regulators on volatiles produced by in vitro grown shoots of Agastache rugosa (Fischer & C.A.Meyer) O. Kuntze Plant Cell Tiss Organ Cult 107:161–167. https://doi.org/10.1007/s11240-011-9954-2
Zielińska S, Dryś A, Piątczak E, Kolniak-Ostek J, Podgórska M, Oszmiański J, Matkowski A (2019) Effect of LED illumination and amino acid supplementation on phenolic compounds profile in Agastache rugosa in vitro cultures. Phytochem Lett 31:12–19
Zielinska S, Wójciak-Kosior M, Płachno BJ, Sowa I, Włodarczyk M, Matkowski A (2018) Quaternary alkaloids in Chelidonium majus in vitro cultures. Ind Crops Prod 123:17–24. https://doi.org/10.1016/j.indcrop.2018.06.062
Zielińska S, Piątczak E, Kromer K (2006) Micropropagation of Lycopus lucidus Turczaninow ex Bentham from shoot tips. In: XI Ogólnopolska Konferencja Kultur in vitro i Biotechnologii Roślin “Kultury in vitro podstawą biotechnologii roślin” Międzyzdroje, 6–9 Września 2006 Roku. XI Ogólnopolska Konferencja Kultur in vitro i Biotechnologii Roślin “Kultury in vitro podstawą biotechnologii roślin” Międzyzdroje, 6–9 Września 2006 Roku
Zych M, Furmanowa M, Krajewska-Patan A, Łowiczka A, Dreger M, Mendlewska S (2005) Micropropagation of Rhodiola kirilowii plants using encapsulated axillary buds and callus. Acta Biol Cracov Bot 47(2):83–87
Acknowledgements
Anastasia Hermosaningtyas participates in the Poznan University of Medical Science STER Internationalization of Doctoral Schools Programs of the NAWA Polish National Agency for Academic Exchange No. PPI/STE/2020/1/0014/DEC/02.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
BT conceptualised the manuscript. BT, MK, and AB surveyed the literature and wrote the first draft of the manuscript. AH prepared the visualisation and edited the manuscript to its final version. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Communicated by Ewa Grzebelus.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thiem, B., Hermosaningtyas, A.A., Budzianowska, A. et al. Polish contributions in developing medicinal plant in vitro propagation system. Plant Cell Tiss Organ Cult 155, 1–28 (2023). https://doi.org/10.1007/s11240-023-02562-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02562-y