Abstract
Avocado, Persea americana Mill, is one of the most traded tropical fruits in the international market. To date, stable and transient transformation has only been achieved for of zygotic embryos and not of adult plant tissue, which limits functional genomics research. We provide the first transient Agrobacterium-mediated transformation methodology in avocado leaves that overcomes the recalcitrance to transformation of this species. We investigated the effect of Agrobacterium strain, leaf stage, wounding pre-treatment, the phytohormone jasmonic acid, and vacuum infiltration on transient transformation of avocado leaves. Using the Agrobacterium strain LBA4404 and the RUBY reporter a transformation frequency of up to 27% was obtained for avocado detached leaves. The transformation efficiency depended on the age of the leaf, with an intermediate stage of leaf development showing the highest efficiency of transient reporter gene expression. Microwounding pre-treatment facilitates agroinfiltration and coupled with leaf age are the primary factors influencing competence for transient transformation. Jasmonic acid did not significantly affect transient transformation in the absence of microwounding. However, microwounding and 250 µM of jasmonic acid acted synergistically to significantly enhance transient expression. Using this methodology with localized vacuum agroinfiltration, transient transformation of attached avocado leaves was achieved. This method unlocks the use of Agrobacterium-mediated transient transformation as a tool for explore gene function and metabolic pathways in both, detached and attached avocado leaves.
Key message
Leaf age, wounding and jasmonic acid counter the recalcitrant nature of avocado, unlocking the use of Agrobacterium mediated transient transformation as a tool for functional genetics in this plant. In addition, localized vacuum infiltration bypasses plant size constraints for in-planta transient transformation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The avocado, Persea americana Mill., is one of the most traded tropical fruits in the international market. Globally, avocado exports tripled between 2010 and 2020 (FAO 2021), and in terms of export value avocado became the leading commodity with 53% of global trade in major tropical fruits in 2021 (FAO 2022). Global demand and lucrative export prices have stimulated the expansion of this horticultural trees crop production.
Despite the great economic importance of the production of this horticultural tree crop, and the recent publication of its genome sequence (Rendón-Anaya et al. 2019), few molecular tools exist or have been utilized for avocado crop improvement (Tamayo-Ramos et al. 2022). Currently the molecular analyses are hindered by the lack of efficient and rapid transformation protocols. In the last two decades there has only been a single report using biolistics for transient transformation of embryogenic callus (Chaparro-Pulido et al. 2014), one report using Agrobacterium rhizogenes to produce composite avocado plants (Prabhu et al. 2017), and few reports detailing the use of Agrobacterium to generate stable transformants (Cruz-Hernández et al. 1998; Efendi 2003; Raharjo et al. 2003, 2008; Palomo-Ríos et al. 2011, 2012, 2017; Ahmed et al. 2012). These latter reports employed embryogenic masses derived from zygotic embryos, due to their high potential and competence for regeneration. However, as these zygotic embryos are derived from outcrossing, they are not the ideal starting material for the preservation of a scion’s agronomic traits.
Whilst stable genetic transformation is essential for crop breeding applications, transient gene transformation, is a useful tool in plant cell and molecular biology research and provides results significantly faster compared to stable transformation. Transient gene expression allows the assessment of gene function and regulation by gene overexpression, protein subcellular localization assays, protein–protein interaction assays (co-immunoprecipitation assay and bimolecular fluorescence complementation assay), as well as protein-DNA interaction (dual luciferase assay) or gene silencing (Lu et al. 2003; Sparkes et al. 2006; Chen et al. 2008; Marion et al. 2008; Zhang et al. 2020). Agrobacterium-mediated transient transformation approaches have proven effective in a wide range of perennial species including aspen (Takata and Eriksson 2012), cacao (Fister et al. 2016), citrus (Acanda et al. 2021), plum (Yanchcva et al. 1994), apple (Maximova et al. 1998) and grapevine (Santos-Rosa et al. 2008). To our knowledge, there are no reports of Agrobacterium-mediated transient transformation of adult avocado plant tissue, perhaps due to avocado’s recalcitrant nature, which limits functional genomics research and breeding (Palomo-Ríos et al. 2012). Tobacco is often used as a model for transient expression studies. However, since some molecular pathways are exclusive to certain species, a heterologous system may not always be appropriate (Manavella and Chan 2009). Therefore, a reliable method for transient gene transformation in avocado plant could significantly improve our ability to perform functional genetic assays in avocado rather than in model species. It could also be used to evaluate the transformation capabilities of different somatic tissues, which then in combination with tissue culture regeneration could identify potential alternatives to somatic embryos for stable avocado transformation.
This research explores different parameters to achieve Agrobacterium-mediated transient genetic transformation in avocado. First, detached leaves were used to evaluate different factors affecting avocado genetic transformation. Among the parameters tested on avocado was forced vacuum infiltration to infiltrate the Agrobacterium suspension (Bechtold and Pelletier 1998); different strains of Agrobacterium tumefaciens (Anderson and Moore 1979); leaf age given that recalcitrance to transformation has been shown to be inversely proportional to tissue age (Cervera et al. 1998); micro wounding of tissues (Potrykus 1990), and pre-culture on phytohormone cocktail-containing medium (Sangwan et al. 1991). Finally, with those optimal conditions identified on detached leaves, we tested in-planta transient gene transformation by vacuum agroinfiltration of leaves of mature avocado plants. We provide the first report of Agrobacterium-mediated transient transformation of detached and attached avocado leaves expressing the genes necessary for the overexpression of an entire biosynthetic pathway not natively found in this species, selecting critical parameters for its success and future application in the study of gene function and genetic improvement of one of the most important fruit crops.
Material and methods
Plant materials
Persea americana Mill. Hass cultivar grafted on Mexican race rootstock was obtained from the Fundación Salvador Sánchez Colín CICTAMEX, S.C. germplasm bank located at La Cruz Experimental Center at Coatepec Harinas in the state of Mexico. Plants were grown under greenhouse conditions at CIATEJ facilities in Zapopan, Jalisco, Mexico (lat. 20°42′N, long. 103°28′W, altitude 1680 m).
Transformation plasmids and Agrobacterium cell cultures
Two plasmids were used in this study to monitor transient gene expression in avocado leaves; pJL-TRBO-G, carrying the Green Fluorescent Protein (GFP) reporter gene, was a gift from John Lindbo (Addgene plasmid # 80083) (Lindbo 2007), and 35S:RUBY, carrying the enzymes required for betalain biosynthesis, was a gift from Yunde Zhao (Addgene plasmid # 160908) (He et al. 2020). Three widely used A. tumefaciens strains AGL1, LBA4404, and GV3101 (Intact Genomics, USA), were evaluated to assess the transient transformation efficiency of avocado leaves, strain EHA105 was not included because it has resistance to the antibiotic spectinomycin as well as the RUBY vector. Each expression vector was transformed into each of the Agrobacterial strain by electroporation. A single colony of transformed A. tumefaciens was plated on Luria–Bertani (LB) plates with the exception of LBA4404 (Yeast malt, YM), with the following antibiotics rifampicin (10 µg/ml) for all strains, carbenicillin (100 µg/ml) for AGL1, streptomycin (50 µg/ml) for LBA4404, gentamycin (30 µg/ml) for GV3101; and for plasmid selection, kanamycin (50 μg/ml) for pJL-TRBO-G, and spectinomycin (50 μg/ml) for 35S:RUBY. Plates were incubated at 28 °C for 48 h in the dark. For agroinfection, colonies of transformed Agrobacterium cells were inoculated into LB broth or YM for LBA4404, supplemented with acetosyringone (AS, 20 µM) and grown 28 °C overnight at 250 RPM. Cultures were harvested by centrifugation at 5000 g for 10 min and pellets were resuspended in infiltration medium (4 g/l Murashige and Skoog Medium (salts and vitamins), 4 g/l sucrose, 20 mM MES, 5 mM MgCl2, 150 µM AS, 0.1% v/v Plant Preservative Mixture (PPM), pH 5.3) (Acanda et al. 2021) before incubating for 4 h at 28 °C at 150 RPM. Cell suspensions were then adjusted to a final optical cell density OD600 of 0.6 with infiltration media. Each treatment applied to detached avocado leaves was also infiltrated into tobacco leaves with a needleless syringe as a positive control.
Agrobacterium mediated transient transformation
In previous studies, the expression levels of both reporters, TRBO-G and RUBY, is sufficient to be visually evaluated (Lindbo 2007; He et al. 2020). For each report gene, expression was visually ranked from 0 to 5, where zero is no expression, and five is high expression compared to the tobacco positive control by observing the leaves at 7 days post infection (DPI). This ranking system was used to initially evaluate the vector, the infiltration method, the Agrobacterium strain, bacterial density and vacuum conditions. Infiltration methods were initially evaluated using three different Agrobacterium strain at a cell density of 0.6 OD600 carrying the viral vector pJL-TRBO-G, or 35S:RUBY, in five avocado detached leaves. Spontaneous infiltration was evaluated by dipping detached leaves into each bacterial suspension for 3, 16, or 24 h, while forced infiltration was tested by needleless syringe and vacuum infiltration. For the latter detached leaves (n = 5) were submerged into each Agrobacterium suspension and were put into a laboratory desiccator connected to a vacuum pump, until the vacuum reached −0.07 MPa; the vacuum was set for 5 min followed by a slow release of vacuum and this procedure repeated twice, in order to replace the air present in intercellular spaces of the leaf tissue with Agrobacterium suspension. Leaves were removed and blotted dry on paper towels to facilitate removal of excess bacterial suspension and air dried for an hour in the laminar airflow of a biosafety cabinet (Fujiuchi et al. 2016). Leaves were then incubated in a semi-wet chamber at 25 °C in the dark for 2 days, then light/dark period of 16/8 h. To evaluate the effect of wounding on transient gene expression, detached avocado leaves were mechanically wounded (60 wounds/cm2) on the abaxial surface of the leaves using a microneedle roller (Supplementary Fig. 1), a device typically used for cosmetic purposes to generate micro-wounds (MW) to facilitate entrance of agrobacteria. To compare the effect of MW on the same avocado leaf, MW was only applied to one half of the leaf. To evaluate the effect of methyl jasmonate (JA) on transient gene expression, prior to vacuum infiltration 250 μM of JA was added to the agrobacterial suspension.
For in-planta transient transformation of avocado, Agrobacterium-mediated vacuum infiltration was applied to attached leaves of a branch, using the optimized conditions previously identified for detached leaves. The same 20 l vacuum desiccator was used, with a built-in analog vacuum gauge at the top of the unit. To allow access of a branch whilst enabling a complete seal, a flexible rubber hose was coated in vacuum grease to fit in the usual grooves on the vacuum chamber and any leaks were sealed using mouldable plastic (e.g., Nescofilm). Leaves were blotted dry on paper towels to facilitate removal of excess bacterial suspension. Attached leaves were then covered with a transparent plastic bag to maintain high humidity and prevent dehydration, plants were incubated at 25 °C in the dark for 2 days, then light/dark period of 16/8 h.
Spectrophotometric detection of Betalains
Agroinfiltrated leaves with RUBY reporter, MW and JA treatments were frozen in liquid nitrogen and ground with a mortar and pestle at 7 DPI. Betalains were extracted from 100 mg of ground tissue by resuspension with 300 µl 50% methanol, 1 mM ascorbic acid, 0.5% formic acid (Grützner et al. 2021). Samples were vortexed and then incubated on ice for 15 min. The samples were centrifuged at 13,000 RPM for 10 min at 4 °C and supernatant was recovered into a new tube. Spectrophotometric determination of betalains was measured at 535 nm (Polturak and Aharoni 2018) using the Infinite 200 PRO microplate reader (TECAN, Switzerland).
Statistical analysis
Absorbance data of betalain extracts were analyzed by one-way ANOVA using Prism 8 software (GraphPad, USA).
Imaging and confocal microscopy
Efficiency of RUBY expression in developmental stage, MW and JA treatments was determined in terms of the percentage of red-stained area compared to the total leaf area by image-J software (NIH, USA). For confocal microscopy a fresh unfixed section of leaf (Approx. 0.5 cm2) was placed under a coverslip for imaging and observed at optical zoom 10X. The fluorescent signals were then detected using a Leica TCS SPE confocal microscope (Leica Microsystems Inc., USA) with a laser excitation at 488 nm and emission filter of 500–530 for GFP expression (Green), and emission filter of 675–725 for chlorophyll autofluorescence (red displayed). Images were taken and managed with the LAS X® software (Leica Microsystems Inc., USA). The GFP fluorescence signal was obtained from detached avocado leaves transiently transformed with the conditions that generated the highest betalain expression.
Protein extraction and Western Blot
Approximately 100 mg of avocado leaves infiltrated with Agrobacterium carrying pJL-TRBO-G following the method that generated the highest betalain expression; leaves were sampled at 7 DPI and flash frozen in liquid nitrogen. For protein extraction, leaf samples were ground into a fine powder and resuspended in 1 ml of TRIzol reagent (Thermo Fisher Scientific, USA) following the manufacturer’s instructions for protein isolation. Total protein extracts were separated by SDS-PAGE in 10% polyacrylamide gels under denaturing conditions. Gels were blotted onto immobilon-P, 0.45 µm PVDF membranes (Merck Millipore, USA). After 3 h blocking with TBS-Tween 0.05% plus 5% fat-free milk, blots were incubated overnight with a monoclonal mouse antibody against GFP (Thermo Fisher Scientific, USA) 1:500 dilution. After washing the membrane three times for 5 min each in 1X TBST, blots were incubated with a goat anti-mouse IgG secondary antibody horseradish peroxidase-conjugated at a 1:5000 dilution (Thermo Fisher Scientific, USA) for 2 h at room temperature. Antibody binding was detected with the Pierce ECL Western blotting Substrate solution, following the manufacturer’s instructions (Thermo Fisher Scientific, USA) and by means of an X-ray film, following standard procedures.
Results
Transient expression of GFP in avocado
In avocado, the use of GFP has been previously reported for transient biolistic transformation of embryogenic callus (Chaparro-Pulido et al. 2014), and for transformation of roots to develop composite avocado plants (Prabhu et al. 2017). In this study, several parameters, e.g., infiltration system, A. tumefaciens strain, Agrobacterium cell density, developmental stage of avocado leaves, pre-treatment with MW, were tested for transient transformation of avocado leaves using a viral vector pJL-TRBO carrying the GFP reporter, but with no success. After 7 DPI, infiltrated and control leaves were examined. However, both negative control (non-transformed) and the agroinfiltrated leaves showed only slight basal fluorescence, under a long wave UV light (365 nm), possibly due to the autofluorescence of the leaf (Buschmann and Lichtenthaler 1998). Supplementary Fig. 2 shows a representative image of negative control and agroinfiltrated leaves. This observation was consistent across different leaves under parameters described above (data not shown) and even after 14 DPI, thus, it was determined that this method was not suitable for rapidly assessing transformation efficiency in avocado. We therefore decided to evaluate the non-fluorescent reporter, RUBY that can be visualized without any special equipment.
Transient expression of the betalain biosynthetic pathway in avocado leaves
We assessed the effect of infiltration system on transient gene expression on avocado detached leaves, using the 35S:RUBY reporter system. This expression vector comprises the cassette for expressing the enzymes P450 oxygenase CYP76AD1, L-DOPA 4,5-dioxygenase and glucosyltransferase, involved in the conversion of tyrosine into the red pigment, betalain (He et al. 2020). The RUBY reporter was selected because it is non-destructive visual reporter that allow simple and cheap macroscopic visual detection of the success of transient genetic transformation, from 3 days after agroinfiltration. We initially determined that avocado leaves are not suitable for agroinfection by co-culture of detached leaves with agrobacteria suspension at 0.6 OD600 for 3, 16, or 24 h as this method induced leaf necrosis after 3 DPI. Agroinfection using a needleless syringe, as is commonly used for agroinfiltration of tobacco, was also found to be not suitable for avocado leaves. We therefore explored the use of vacuum infiltration of the bacterial suspension to evaluate the effect of Agrobacterium strain on transient gene expression. Since A. tumefaciens strains have different sensitivities to particular plant species, we started by testing three of the most commonly used laboratory strains (AGL1, LBA4404, and GV3101). Microwounding of one half of each leaf was performed prior to vacuum infiltration with five detached young leaves of avocado subjected to vacuum-based agroinfiltration at −0.07 MPa for 5 min with three vacuum breaks, with each strain suspension at 0.6 OD600.. Following vacuum infiltration, leaves were incubated in a semi-wet chamber at 25 °C, and RUBY expression was evaluated at 7 DPI. At this stage, the accumulation of betalains was visually evident, mainly around the microneedle-made wounds, as shown in Fig. 1. Different parameters (agrobacterial strains, bacterial density and vacuum conditions) were initial screened by macroscopic visual detection, on a scale we assigned from 0 to 5, by comparing the result in avocado leaf to the positive control in tobacco. Among the three Agrobacterium strains assessed, transient expression of RUBY was greatest with strain LBA4404 and lowest with AGL1 (Supplementary Table 1). This result shows that vacuum-based agroinfiltration is suitable for agroinfection of avocado leaves and that heterologous expression of the RUBY reporter functionally re-constitutes the betalain biosynthetic pathway in avocado leaves. However, these results also demonstrate that Agrobacterium strain influences transformation efficiency.
Effect of leaf stage and microwounding on expression of RUBY. Representative image of one of five biological replicates of RUBY expression in avocado leaves at stages C-E and with microwounding applied to one half of the leaf (+ MW), or without microwounding (−MW). Betalain (red) is located around the microneedle-made wounds. (Color figure online)
We next explored the optimal vacuum conditions and bacterial density for agroinfiltration of avocado leaves. Three different vacuum intensities (−0.02, −0.04, and −0.07 MPa), three different durations (1, 2, or 3 vacuum breaks), and three different cell densities (OD600 values of 0.3, 0.6, 1.0), were evaluated by monitoring betalain staining at 7 DPI. Betalain staining positively correlated with increasing vacuum intensity and the number of vacuum breaks suggesting the intensity and repetitions of vacuum infiltration has an effect on transformation efficiency (Supplementary Table 2). The vacuum infiltration conditions of −0.07 MPa, for 5 min with 3 vacuum breaks was then used to determine the effect of cell density on transient transformation using the LBA4404 Agrobacterium strain (OD600 0.3, 0.6 and 1). Using the visual scoring system, it was determined that an OD600 of 0.6 resulted in higher betalain staining (Supplementary Table 3).
Effect of leaf age on transient gene expression
We next assessed the effect of leaf age on transient transformation using the optimized conditions previously identified (LBA4404 strain at 0.6 OD600, leaf microwounding, vacuum infiltration at −0.07 MPa with 3 vacuum breaks). To assess the effect on Agrobacterium-mediated transformation of the developmental stage of avocado leaves, five successive developmental stages of leaves were selected based on macroscopic characteristics of size and color changes. The latter due to the greening pattern common in many tropical species due to the gradual increase in chlorophyll content (Whatley 1992). The first stage (A) corresponds to emerging leaves, the shortest in length in this classification, thin and bright brown in color; followed by leaves that undergo expansion but are of the same colour (B); then those that continue to expand in size but show an evident color transition towards green (C), then those that are light green and less thin than the previous ones (D) and finally the fully expanded, dark green and thick leaves (E) (Fig. 2). Leaves at the earliest stages (A and B) did not survive the infiltration treatments and so were excluded from our analysis. In contrast, after 7 DPI leaves at stage C and D displayed betalain in the periphery of the microneedle-made wounds but not in the non-wounded side of the leaves (Fig. 1), indicating the crucial impact of MW in transformation efficiency. Older leaves (stage E) consistently showed no evidence of reporter expression even after 14 DPI (Fig. 3).
Representative image of different avocado leaf stages used in the study: Detached leaves were grouped as follows, the first stage (A) corresponds to emerging leaves, the shortest in length in this classification, thin and bright brown in color; followed by leaves that undergo expansion but are of the same color (B); then those that continue to expand in size but show an evident color transition towards green (C), then those that are light green and less thin than the previous ones (D) and finally the fully expanded, dark green and thick leaves (E). Scale bar 2.5 cm. (Color figure online)
Effect of wounding pre-treatment and jasmonic acid
Previous work has shown that pre-treatment with the phytohormone JA can affect Agrobacterium mediated transformation (Jung et al. 2014). Having determined that MW and leaf age are critical factors, we therefore evaluated whether treatment with JA affects the efficiency of transient expression. Previous studies on detached sunflower leaves achieved a 4.6-fold increase in heterologous expression when JA 250 mM was added to A. tumefaciens cell suspensions and infiltrated into the plant leaves (Jung et al. 2014; Jung and McDonald 2016). Our observations were focused on leaves at stage C and D and using agroinfiltration of the RUBY reporter. Here, there was no significant betalain synthesis in leaves treated only with JA, and without MW, demonstrating that MW coupled with leaf age are the primary factors influencing the competence for transient transformation (Fig. 4).
Effect of MW and JA. Betalain accumulation following agroinfiltration of avocado leaves (stage C and D) with the RUBY reporter. Leaves represent independent agroinfiltrations for each treatment. Top row, no MW plus JA (250 µM) (−MW, + JA); middle row, MW without JA (+ MW, −JA); and bottom row, MW plus JA (250 µM) treatment (+ MW, + JA)
Whilst JA did not appear to significantly affect transient transformation in the absence of MW, we did observe a synergistic effect when leaves were both MW and JA treated, which was consistent for both C and D leaf stages (Fig. 4). Image analysis showed that betalains accumulation was highest in stage D, followed by stage C leaves with MW and JA treatments, with betalains accumulation constituting 27.9% of total leaf area and 12.1%, respectively. In those treatments with MW but without JA the detected area of betalain staining is less than 10% being again higher for stage D, followed by C with 9.3% and 6%, respectively. Betalains are natural water-soluble pigments composed of betaxanthins and betacyanins, and show highest absorption at the 460–480 nm and 535–538 nm range (Polturak and Aharoni 2018). The image analysis data of leaf area accumulating betalains was supported by spectrophotometric quantification of leaf extracts (Fig. 5). Betalain was produced and accumulated at a significantly greater level in leaves at stage C and D with MW and JA (+ MW, + JA) treatment.
Confocal microscopy of GFP- expressing avocado leaves with synergic effect of MW and JA
Since betalain biosynthesis was successfully observed by agroinfiltration of D-stage avocado leaves with MW pre-treatment and addition of JA, we tried again to assess GFP expression this time by confocal microscopy. After infiltration of agrobacteria harboring the pJL-TRBO-G into detached avocado leaves, we assessed GFP fluorescence as an indicator of successful Agrobacterium-mediated transformation. Apparent GFP fluorescence was mainly observed around some of the wounds (Fig. 6). The localization pattern of GFP around the wounds is similar to that observed with RUBY. Moreover, we also confirmed the accumulation of GFP protein by western blot of total protein extracts with monoclonal anti-GFP antibody. Western blot analysis revealed that MW in combination with JA treatment resulted in detectable GFP expression in some but not in all independent samples. No GFP was detected in leaves that were treated with JA but not MW (Fig. 7).
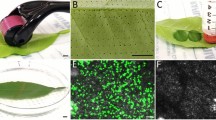
Confocal microscopy of avocado leaves infiltrated with pJL-TRB-G. Fluorescence imaging of avocado leaves with MW (center, hole) and JA (250 µM) in negative control (A–C) and transformed with pJL-TRBO-G (D–F). GFP fluorescence (A, D), chlorophyll fluorescence (B, E), and merged images (C, F). Scale bar: 100 μm, Optical zoom: 10X
Detection of GFP in agroinfiltrated avocado leaves. Total protein was extracted from independent leaves agroinfiltrated with pJL-TRBO-G. Lanes 1–4, independent plants were microwounded and treated with JA (250 µM); lanes 5–8 leaves were treated with JA only. Recombinant GFP was used as positive control ( +)
Vacuum-based transformation In-Planta
Since conventional in-planta transient transformation techniques such as floral dip, and needleless syringe infiltration are not an alternative for many perennial species, we assessed whether the methods optimized for expression in detached leaves could be adapted for in-planta transient transformation. A major limitation here is that plant size prevents whole plant agroinfiltration with conventional infiltration vessels too small for this purpose. In this study, due to the larger size of the plant compared to the vacuum chamber, we applied the vacuum-based agroinfiltration to avocado attached leaves of a branch and not to the whole plant. To allow access of a plant branch inside the vacuum chamber a flexible rubber hose coated with vacuum grease was used to provide the vacuum seal between the upper and lower halves of the desiccator (Fig. 8a). The small gaps between the branch and the adapter must be sealed to allow vacuum formation inside the desiccator. This made it possible for several leaves attached to a branch of an avocado plant to be immersed in the Agrobacterium suspension and subjected to vacuum infiltration. Young leaves (stage C and D) attached to a branch of 1.7 m tall avocado plants were subjected to vacuum-based agroinfiltration at −0.07 MPa intensity together with 3 vacuum breaks, with LBA4404 strain at 0.6 OD600, along with a pre-treatment of both MW and 250 μM JA. At 7 DPI, all infiltrated leaves showed betalain staining, with the same localization pattern as observed for detached leaves (Fig. 8b).
Agrobacterium-mediated in-planta transient transformation of avocado with the RUBY reporter. A Vacuum-based agroinfiltration system with a vacuum pump and desiccator using a flexible rubber hose that has been coated in vacuum grease to fit in the usual grooves on the vacuum chamber, as a seal to allow access of a plant branch inside the vacuum chamber between the upper and lower halves of the desiccator and avoid branch crushing and vacuum loss; B Betalain accumulation in leaves attached to the branch of avocado plants at 7 DPI
Discussion
In model plants such as tobacco transformed with GFP constructs, the use of a longwave UV lamp has been demonstrated to be a rapid means of evaluating transformation (Casper and Holt 1996)however, it has been reported that the detection of GFP in green tissues using this macroscopic method can lead to a substantial underestimation of expression levels. In this study, our inability to detect GFP fluorescence using this method could be due to the low transformation efficiency of the pJL-TRBO-G construct or interference by pigments within avocado leaves. Although it is a vector capable of expressing very high levels of foreign proteins in N. benthamiana plants, it has previously been reported to be unsuccessful in transforming detached whole sunflower, a plant that does not belong to the Solanaceae family (Jung et al. 2014). In addition, chlorophyll fluorescence has been shown to interfere with the detection of GFP fluorescence in Medicago, rice and Arabidopsis, (Zhou et al. 2005). Our results indicate that GFP it is not a suitable reporter for rapid macroscopic evaluation by long wavelength UV illumination in avocado leaves and instead detection of this reporter requires access to specialized equipment.
The main advantage of RUBY is that visualizing the transformed area does not require specialized equipment such as fluorescence or confocal microscopes, contributing to the optimization of transient transformation methods in a quick and easy way, monitoring the plant throughout its life cycle. The RUBY reporter system has only recently been developed and has so far been used as a visible marker in Arabidopsis, rice (He et al. 2020), and Bamboo (Chen et al. 2021) and is promising to drive the evaluation of plants with thick waxy cuticles. The bright red color from betalain is easily contrasted from green leaf color and the brown caused by leaf damage and deterioration. However, this reporter does not allow detection at the subcellular level, as GFP does. The RUBY cassette was functionally expressed in avocado leaves, facilitating the observation of transient transformation, due to the production of functional enzymes for betalain synthesis, which led to the production of red spots on avocado leaves easily distinguishable with the naked eye from the third day after agroinfiltration.
Preliminary experiments indicated that avocado leaves are not suitable for agroinfection by co-culture with Agrobacterium, which is consistent with previous reports that reported tissue necrosis on leaf-discs of grape after co-cultivation with the LBA4404 Agrobacterium strain (Das et al. 2002). The vacuum-based agroinfiltration of detached leaves allowed testing conditions for transient expression in a more time and cost-effective manner than stable transformation. For avocado leaves where spontaneous infiltration or forced infiltration using needle-less syringe is not possible, vacuum infiltration allows controlled introduction of bacterial suspensions into leaf tissues, overcoming the influence of leaf structure on transformation efficiency of avocado leaves. One disadvantage is that this method requires specialized equipment such as a vacuum chamber with a pressure gauge, connected to a pump. Our results of increased expression of the RUBY reporter in avocado leaves by using −0.07 MPa vacuum intensity, and three vacuum breaks agrees with previous data possibly through increased of Agrobacterium cell suspension into leaf tissue (Joh et al. 2005; Simmons et al. 2009).
The macroscopic visual detection method of reporters provides a rapid way of screening for successful transient gene transformation events. Among the three Agrobacterium strains assessed, we observed that the LBA4404 is suitable for transient expression in avocado leaves. In two previous avocado transformation studies comparing different Agrobacterium strains, the LBA4404 strain did not give the best results, though this may be because the tissue they agroinfiltrated were globular somatic embryonic masses (Palomo-Ríos et al. 2012), and embryonic shoot axes (Ahmed et al. 2012).
We have determined that avocado leaves within a relatively narrow developmental time span are required for Agrobacterium-mediated transient genetic transformation, even with the addition of vacuum, MW and the application of JA. Working with mature leaves should be avoided until conditions for this leaf stage are optimized. It has been demonstrated that the transformation of plant cells by Agrobacterium is inversely proportional to the age of the tissue (Wixom et al. 2018). The evaluation of different stages of leaf development of the tropical tree Theobroma cacao L. for transient transformation with Agrobacterium has shown that leaf age affected transformation efficiency (Fister et al. 2016). Some authors have proposed that physiological changes in the leaves when they mature hinder the infiltration of the Agrobacterium cell culture into the leaf parenchyma, therefore by reducing the diffusion of the Agrobacterium suspension and hence limiting the transformation potential of the tissue. The diffusive capability of syringe-infiltrated Agrobacterium suspension has been associated with the volume of the intercellular air spaces and the arrangement of the mesophyll cells inside the leaves (Zheng et al. 2021). A small intercellular and compartmentalized space, together with compact mesophyll cells could restrict the spread of the infiltrated suspension and therefore the transformation of cells. In Rosa chinensis it is thought that the stratum corneum and wax coat present in the outermost layer of mature leaves are responsible for the difficulty in infiltration and therefore the low transformation mediated by Agrobacterium (Lu et al. 2017). Another possible explanation for the interesting result for the null transformation efficiency of leaves in the E group is that we hypothesize that at the time of making the micro-holes with the MW, the dense waxy layer covered the hole created by the needle and this prevented agroinfiltration, due to the fact that 0% transformation efficiency in all leaves is quite peculiar in this leaf stage regardless of the treatments, leading to speculation as to whether the Agrobacterium suspension really did infiltrate.
Wounding is an integral step in Agrobacterium-mediated transformation and as well as providing an entry point for the bacteria, activates the production of vir-inducing molecules that facilitate the successful transformation of plants (Horsch et al. 1985). In avocado, the generation of micro-injuries on the leaf surface has a positive effect on transient gene expression, as has been reported for citrus (Acanda et al. 2021). Since other phenolic compounds such as vanillin and cinnamic acid have been previously reported to induce vir genes even more potently than acetosyringone (Cha et al. 2011), and given that these compounds are present in avocado (Castro-López et al. 2019), we hypothesized that the phenolic compounds released by the avocado leaf as a result of the MW pre-treatment, together with acetosyringone pre-treatment, may be behind the improved transformation efficiency.
Our result has shown for first time that MW pre-treatment and JA acted synergistically, and both were necessary to significantly improve avocado genetic transformation. Plant recalcitrance to Agrobacterium transformation is generally attributed to the activation of plant immune responses upon perception of the bacterium (Pitzschke 2013). JA is a phytohormone that signals the plant defense response to insect injury by activating induced systemic resistance (ISR), at the same time deactivating systemic acquired resistance (SAR), which is triggered by salicylic acid (SA) and defends the plant against biotrophic infections. Therefore, addition of JA to the Agrobacterium infiltration medium is hypothesized to suppress SAR, by complex crosstalk between JA and SA, making the avocado leaves more vulnerable to bacterial infections (Pieterse et al. 2009). Plants deficient in SA have shown increased susceptibility to Agrobacterium, while plants overproducing this metabolite show increased recalcitrance to infection (Yuan et al. 2007). A study of JA application prior to agroinfiltration of Nicotiana benthamiana leaves (Robert et al. 2015) suggests that JA has practical utility for enhancing recombinant protein expression by producing a significant depletion of large and small subunit of RuBisCO, and consequently an availability of metabolites and cellular resources for recombinant proteins. This synergistic response may be due to all the individual conditions mentioned above and as best of our knowledge has not been previously reported for avocado, opening an encouraging perspective for functional gene studies. It is unclear the effect of other concentrations promoted avocado leaves transformation, as this was not examined.
Agroinfiltration of attached leaves, rather than detached leaves, is ideal for further phenotypic experiments. Thus, the protocol of vacuum-based agroinfiltration of attached leaves to a plant larger than the vacuum chamber size unlocks the use of Agrobacterium mediated in-planta transient transformation as a tool for functional genetics in avocado. Using this method can open the way to explore gene function and metabolic pathways in this non-model plant and can be a starting point for other recalcitrant species to transformation. Compared to other methods of transient gene transformation, the vacuum-based agroinfiltration method for transient gene overexpression in avocado leaves is less time-consuming than protoplasts-based genetic transformation, is less expensive than biolistics, and does not have the compatibility, and gene of interest size limitations that viral vectors can have. To our knowledge, the vacuum-based agroinfiltration method has not been reported in a localized part of a plant that exceeds the size of the vacuum infiltration chamber, opening the possibility to apply in-planta transient transformation without being limited by plant size. If this can be coupled to the regeneration of transformed cells either in leaves or other somatic cells, this would provide an opportunity to generate stable transgenic lines for clonally propagated cultivars, which could offer significant opportunity for trait improvement.
Data availability
Enquiries about data availability should be directed to the authors.
References
Acanda Y, Welker S, Orbović V, Levy A (2021) A simple and efficient agroinfiltration method for transient gene expression in Citrus. Plant Cell Rep 40:1171–1179. https://doi.org/10.1007/s00299-021-02700-w
Ahmed MF, Kantharajah AS, Holford P (2012) Genetic transformation studies on avocado cultivar “Hass” (Persea americana). Am J Plant Sci 03:1225–1231. https://doi.org/10.4236/ajps.2012.39148
Anderson AR, Moore LW (1979) Host specificity in the genus Agrobacterium. Phytopathology 32:320–323. https://doi.org/10.1094/Phyto-69-320
Bechtold N, Pelletier G (1998) Of adult arabidopsis thaliana plants by vacuum infiltration. Methods 82:259
Buschmann C, Lichtenthaler HK (1998) Principles and characteristics of multi-colour fluorescence imaging of plants. J Plant Physiol 152:297–314. https://doi.org/10.1016/S0176-1617(98)80144-2
Casper SJ, Holt CA (1996) Expression of the green fluorescent protein-encoding gene from a tobacco mosaic virus-based vector. Gene 173:69–73. https://doi.org/10.1016/0378-1119(95)00782-2
Castro-López C, Bautista-Hernández I, González-Hernández MD et al (2019) Polyphenolic profile and antioxidant activity of leaf purified hydroalcoholic extracts from seven mexican Persea americana Cultivars. Molecules. https://doi.org/10.3390/molecules24010173
Cervera M, Juárez J, Navarro A et al (1998) Genetic transformation and regeneration of mature tissues of woody fruit plants bypassing the juvenile stage. Transgenic Res 7:51–59. https://doi.org/10.1023/A:1008855922283
Cha TS, Chen CF, Yee W et al (2011) Cinnamic acid, coumarin and vanillin: alternative phenolic compounds for efficient Agrobacterium-mediated transformation of the unicellular green alga, Nannochloropsis sp. J Microbiol Methods 84:430–434. https://doi.org/10.1016/j.mimet.2011.01.005
Chaparro-Pulido CA, Montiel MM, Palomo-Ríos E et al (2014) Development of an efficient transient transformation protocol for avocado (Persea americana Mill.) embryogenic callus. Vitr Cell Dev Biol—Plant 50:292–298. https://doi.org/10.1007/s11627-013-9564-2
Chen H, Zou Y, Shang Y et al (2008) Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol 146:368–376. https://doi.org/10.1104/pp.107.111740
Chen K, Hu K, Xi F et al (2021) High-efficient and transient transformation of oso bamboo (Phyllostachys edulis) and Ma bamboo (Dendrocalamus latiflorus Munro). J Plant Biol. https://doi.org/10.1007/s12374-020-09294-y
Cruz-Hernández A, Witjaksono LRE, Gomez-Lim M (1998) Agrobacterium tumefaciens—mediated transformation of embryogenic avocado cultures and regeneration of somatic embryos. Plant Cell Rep 17:497–503. https://doi.org/10.1007/s002990050431
Das D, Reddy M, Upadhyaya K, Sopory S (2002) An efficient leaf-disc culture method for the regeneration via somatic embryogenesis and transformation of grape (Vitis vinifera L.). Plant Cell Rep 20:999–1005. https://doi.org/10.1007/s00299-002-0441-4
Efendi D (2003) Transformation and cryopreservation of embryogenic avocado (Persea americana Mill.) cultures. University of Florida
FAO (2021) Major tropical fruits statistical compendium 2020. Rome
FAO (2022) Major tropical fruits: preliminary results 2021. Rome
Fister AS, Shi Z, Zhang Y et al (2016) Protocol: transient expression system for functional genomics in the tropical tree Theobroma cacao L. Plant Methods 12:1–13. https://doi.org/10.1186/s13007-016-0119-5
Fujiuchi N, Matsuda R, Matoba N, Fujiwara K (2016) Removal of bacterial suspension water occupying the intercellular space of detached leaves after agroinfiltration improves the yield of recombinant hemagglutinin in a Nicotiana benthamiana transient gene expression system. Biotechnol Bioeng 113:901–906. https://doi.org/10.1002/bit.25854
Grützner R, Schubert R, Horn C et al (2021) Engineering betalain biosynthesis in tomato for high level betanin production in fruits. Front Plant Sci 12:1–17. https://doi.org/10.3389/fpls.2021.682443
He Y, Zhang T, Sun H et al (2020) A reporter for noninvasively monitoring gene expression and plant transformation. Hortic Res 7:1–6. https://doi.org/10.1038/s41438-020-00390-1
Horsch RB, Fry JE, Hoffmann NL et al (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Joh LD, Wroblewski T, Ewing NN, VanderGheynst JS (2005) High-level transient expression of recombinant protein in lettuce. Biotechnol Bioeng 91:861–871. https://doi.org/10.1002/bit.20557
Jung SK, Lindenmuth BE, Mcdonald KA et al (2014) Agrobacterium tumefaciens mediated transient expression of plant cell wall-degrading enzymes in detached sunflower leaves. Biotechnol Prog 30:905–915. https://doi.org/10.1002/btpr.1888
Jung SK, McDonald KA (2016) Improved transient production of a cellulase enzyme in detached sunflower leaves using plant hormones. Biotechnol Bioprocess Eng 21:726–732. https://doi.org/10.1007/s12257-016-0410-3
Lindbo JA (2007) TRBO: A high-efficiency tobacco mosaic virus RNA-based overexpression vector. Plant Physiol 145:1232–1240. https://doi.org/10.1104/pp.107.106377
Lu R, Martin-Hernandez AM, Peart JR et al (2003) Virus-induced gene silencing in plants. Methods 30:296–303. https://doi.org/10.1016/S1046-2023(03)00037-9
Lu J, Bai M, Ren H et al (2017) An efficient transient expression system for gene function analysis in rose. Plant Methods. https://doi.org/10.1186/s13007-017-0268-1
Manavella PA, Chan RL (2009) Transient transformation of sunflower leaf discs via an agrobacterium-mediated method: applications for gene expression and silencing studies. Nat Protoc 4:1699–1707. https://doi.org/10.1038/nprot.2009.178
Marion J, Bach L, Bellec Y et al (2008) Systematic analysis of protein subcellular localization and interaction using high-throughput transient transformation of Arabidopsis seedlings. Plant J 56:169–179. https://doi.org/10.1111/j.1365-313X.2008.03596.x
Maximova SN, Dandekar AM, Guiltinan MJ (1998) Investigation of Agrobacterium-mediated transformation of apple using green fluorescent protein: high transient expression and low stable transformation suggest that factors other than T-DNA transfer are rate-limiting. Plant Mol Biol 37:549–559. https://doi.org/10.1023/A:1006041313209
OECD/FAO (2021) OECD-FAO agricultural outlook 2021–2030. OECD Publishing, Paris
Palomo-Ríos E, Barceló M, Barceló-Muñoz A et al (2011) Transformación vía Agrobacterium tumefaciens para inducir tolerancia a la podredumbre blanca del aguacate. Proc VII World Avocado Congress 2011:1–7
Palomo-Ríos E, Barceló-Muñoz A, Mercado JA, Pliego-Alfaro F (2012) Evaluation of key factors influencing Agrobacterium-mediated transformation of somatic embryos of avocado (Persea americana Mill.). Plant Cell Tissue Organ Cult 109:201–211. https://doi.org/10.1007/s11240-011-0086-5
Palomo-Ríos E, Cerezo S, Mercado JA, Pliego-Alfaro F (2017) Agrobacterium-mediated transformation of avocado (Persea americana Mill.) somatic embryos with fluorescent marker genes and optimization of transgenic plant recovery. Plant Cell Tissue Organ Cult 128:447–455. https://doi.org/10.1007/s11240-016-1122-2
Pieterse CMJ, Leon-reyes A, Van Der ES, Van Wees SCM (2009) Networking by small-molecule hormones in plant immunity. Nat Chem Biol 5:308–316. https://doi.org/10.1038/nchembio.164
Pitzschke A (2013) Agrobacterium infection and plant defense-transformation success hangs by a thread. Front Plant Sci 4:1–12. https://doi.org/10.3389/fpls.2013.00519
Polturak G, Aharoni A (2018) “La Vie en Rose”: biosynthesis, sources, and applications of betalain pigments. Mol Plant 11:7–22. https://doi.org/10.1016/j.molp.2017.10.008
Potrykus I (1990) Gene transfer to cereals: an assessment. Nat Biotechnol 8:535–542. https://doi.org/10.1038/nbt0690-535
Prabhu SA, Ndlovu B, Engelbrecht J, Van Den Berg N (2017) Generation of composite Persea americana (Mill.) (avocado) plants: a proof-of-concept-study. PLoS ONE 12:1–22. https://doi.org/10.1371/journal.pone.0185896
Raharjo SHT, Witjaksono NFN, Gomez-Lim MA et al (2008) Recovery of avocado (Persea americana Mill.) plants transformed with the antifungal plant defensin gene PDF1.2. Vitr Cell Dev Biol—Plant 44:254–262. https://doi.org/10.1007/s11627-008-9117-2
Raharjo S, Witjaksono, Efendi D, et al (2003) Genetic transformation of avocado. In Proceedings V World Avocado Congress, pp 115–118
Rendón-Anaya M, Ibarra-Laclette E, Méndez-Bravo A et al (2019) The avocado genome informs deep angiosperm phylogeny, highlights introgressive hybridization, and reveals pathogen-influenced gene space adaptation. Proc Natl Acad Sci USA 116:17081–17089. https://doi.org/10.1073/pnas.1822129116
Robert S, Goulet MC, D’Aoust MA et al (2015) Leaf proteome rebalancing in Nicotiana benthamiana for upstream enrichment of a transiently expressed recombinant protein. Plant Biotechnol J 13:1169–1179. https://doi.org/10.1111/pbi.12452
Sangwan RS, Bourgeois Y, Sangwan-Norreel BS (1991) Genetic transformation of Arabidopsis thaliana zygotic embryos and identification of critical parameters influencing transformation efficiency. MGG Mol Gen Genet 230:475–485. https://doi.org/10.1007/BF00280305
Santos-Rosa M, Poutaraud A, Merdinoglu D, Mestre P (2008) Development of a transient expression system in grapevine via agro-infiltration. Plant Cell Rep 27:1053–1063. https://doi.org/10.1007/s00299-008-0531-z
Simmons CW, Vandergheynst JS, Upadhyaya SK (2009) A model of Agrobacterium tumefaciens vacuum infiltration into harvested leaf tissue and subsequent in planta transgene transient expression. Biotechnol Bioeng 102:965–970. https://doi.org/10.1002/bit.22118
Sparkes IA, Runions J, Kearns A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1:2019–2025. https://doi.org/10.1038/nprot.2006.286
Takata N, Eriksson ME (2012) A simple and efficient transient transformation for hybrid aspen (Populus tremula × P. tremuloides). Plant Methods 8:1–10. https://doi.org/10.1186/1746-4811-8-30
Tamayo-Ramos DI, Salazar-González JA, Casson SA, Urrea-López R (2022) Old and new horizons on Persea americana transformation techniques and applications. Plant Cell Tissue Organ Cult 150:253–266. https://doi.org/10.1007/s11240-022-02268-7
Whatley JM (1992) Plastid development in distinctively coloured juvenile leaves. New Phytol 120:417–426. https://doi.org/10.1111/j.1469-8137.1992.tb01082.x
Wixom AQ, Casavant NC, Kuhl JC et al (2018) Solanum sisymbriifolium plants become more recalcitrant to Agrobacterium transfection as they age. Physiol Mol Plant Pathol 102:209–218. https://doi.org/10.1016/j.pmpp.2018.03.004
Yanchcva SD, Vlahova M, Gercheva P, Atanassov A (1994) Agrobacterium mediated transient expression of p-glucuronidase gus gene in plum (Prunus domestica l.). Biotechnol Biotechnol Equip 8:12–13. https://doi.org/10.1080/13102818.1994.10818763
Yuan ZC, Edlind MP, Liu P et al (2007) The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci USA 104:11790–11795. https://doi.org/10.1073/pnas.0704866104
Zhang Y, Chen M, Siemiatkowska B et al (2020) A highly efficient agrobacterium-mediated method for transient gene expression and functional studies in multiple plant species. Plant Commun 1:100028. https://doi.org/10.1016/j.xplc.2020.100028
Zheng L, Yang J, Chen Y et al (2021) An improved and efficient method of Agrobacterium syringe infiltration for transient transformation and its application in the elucidation of gene function in poplar. BMC Plant Biol 21:1–19
Zhou X, Carranco R, Vitha S, Hall TC (2005) The dark side of green fluorescent protein. New Phytol 168:313–322. https://doi.org/10.1111/j.1469-8137.2005.01489.x
Acknowledgements
We thank Engr. Diana Isabel Tamayo-Ramos for her support with the image analysis with image-J, and Dr. José Juvencio Castañeda-Nava for assistance with confocal microscopy. We also thank CIATEJ and Laboratorio Nacional PlanTECC, México, for facility support. M.C.M. (CVU: 1108072) and L.E.B.R. (CVU: 1090641) conducted their master studies with funding from the Consejo Nacional de Ciencia y Tecnología, México (CONACyT). R.U.L. acknowledges support from Consejo Estatal de Ciencia y Tecnología de Jalisco (COECYTJAL), and Secretaría de Innovación Ciencia y Tecnología (SICYT), Jalisco, México (Grant 7270-2018). S.A.C acknowledge support from BBSRC (BB/S012850/1).
Funding
This work was supported by Consejo Estatal de Ciencia y Tecnología de Jalisco (COECYTJAL) and Secretaría de Innovación Ciencia y Tecnología (SICYT), Jalisco, Mexico, (Newton Fund, Grant 7270-2018). S.A.C. was supported by funding from the Biotechnology and Biological Sciences Research Council (BBSRC) grant to (BB/S012850/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
JASG, MCM, LEBR, and EMT performed the experiments and analyzed the data. RUL and SAC were responsible for the idea of the article, the funding acquisition and project administration. All authors contributed to the writing-reviewing and editing.
Corresponding authors
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Additional information
Communicated by Ming-Tsair Chan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salazar-González, J.A., Castro-Medina, M., Bernardino-Rivera, L.E. et al. In-planta transient transformation of avocado (Persea americana) by vacuum agroinfiltration of aerial plant parts. Plant Cell Tiss Organ Cult 152, 635–646 (2023). https://doi.org/10.1007/s11240-022-02436-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02436-9