Abstract
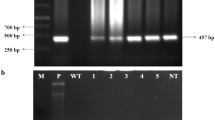
Pigeonpea is an important food legume crop cultivated in tropical and sub-tropical regions around the world wherein the Indian subcontinent accounting for over 90 % of global production. It is a rich source of protein and is an important component of a well-balanced diet for the majority of Indians. Among the other insect pests, pigeonpea productivity is mostly affected by Helicoverpa armigera which is causing severe yield loss. Non-availability of resistant genes in germplasm and constraints with traditional breeding induce the application of a genetic engineering approach to generate insect resistance in pigeonpea. Expression of plant defensins in various crops provided enhanced resistance towards a variety of pests and pathogens. In the current study, two defensins Trigonella foenum-graecum defensin 2 (Tfgd2) and Raphanus sativus antifungal protein 2 (RsAFP2) integrated by a linker peptide was transferred into pigeonpea as a fusion gene by Agrobacterium mediated transformation. Putative transgenic lines were confirmed through PCR and the promising lines were identified in the following generations based upon integration, expression and bioefficiency of the fusion gene. Leaf bioassay conducted against H. armigera larvae showed increased levels of insect resistance compared to the control, where six T2 plants were identified as superior lines showing less than 25 % of leaf damage. Our findings illustrates that Tfgd2–RsAFP2 fusion protein is efficient in imparting protection against the insect pest and the transgenic lines developed in this study could be used for further pigeonpea improvement projects.
Key message
Over-expression of Tfgd2-RsAFP2 fusion gene conferred enhanced insect resistance against Helicoverpa armigera in transgenic pigeonpea plants.








Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. Data derived supporting the findings of this study are available from the corresponding author on request.
References
Bezirganoglu I, Hwang S, Fang TJ, Shaw JF (2013) Transgenic lines of melon (Cucumis melo L. var. makuwa cv. ‘Silver Light’) expressing antifungal protein and chitinase genes exhibit enhanced resistance to fungal pathogens. Plant Cell Tiss Organ Cult 112:227–237
Bizly SP, Rugh CL, Meagher RB (2000) Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nat Biotechnol 18:213–217
Carvalho AO, Gomes VM (2009) Plant defensins – prospects for the biological functions and biotechnological properties. Peptides 30:1007–1020
Carvalho A, Moreira Gomes V (2011) Plant defensins and defensin-like peptides-biological activities and biotechnological applications. Curr Pharm Des 17(38):4270–4293
Chen SC, Liu AR, Wang FH, Ahammed GJ (2009) Combined overexpression of chitinase and defensin genesin transgenic tomato enhances resistance to Botrytis cinerea. Afr J Biotechnol 8:5182–5188
Choudhary AK, Raje SR, Datta S, Sultana R, Ontagodi T (2013) Conventional and molecular approaches towards genetic improvement in pigeon pea for insects resistance. Am J Plant Sci 4:372–385. https://doi.org/10.4236/ajps.2013.42A049
Das A et al (2016) Expression of chimeric Bt gene, Cry1Aabc in transgenic pigeon pea (cv. Asha) confers resistance to gram pod borer (Helicoverpa armigera Hubner.). Plant Cell Tiss Org Cult 127:705–715
Dayal S, Lavanya M (2003) An efficient protocol for shoot generation and genetic transformation of pigeonpea (Cajanus cajan (L.) Millsp.) using leaf explants. Plant Cell Rep 21:1072–1079. https://doi.org/10.1007/s00299-003-0620-y
De Bondt A, Zaman S, Broekaert W, Cammue B, Keulemans J (1999) Genetic transformation of apple (Malus pumila Mill.) for increased fungal resistance, in vitro antifungal activity in protein extracts of transgenic apple expressing Rs-AFP2 or Ace-AMP1. Acta Hort 484:565–570
Dunwell JM (2000) Transgenic approaches to crop improvement. J Exp Bot 51:487–496
Faostat F (2016) Agriculture organization of the united nations statistics division. Economic and Social Development Department, Rome, Italy. Available online: http://faostat3.fao.org/home/E. Accessed 31 Dec 2016
FAO Statistics (2020) Food and Agriculture Organization of the United Nations, Rome http://faostat.fao.org. Accessed 17 May 2020
FAO (2014) Agriculture, Forestry and Other Land Use Emissions by Sources and Removals by Sinks: 1990–2011 Analysis. FAO Statistics Division Working Paper Series, 14/01. UN FAO, Rome, Italy. Available at: http://www.fao.org/docrep/019/i3671e/i3671e.pdf. Accessed 1 Sept 2014
Francois IEJA, Hemelrijck WV, Aerts AM, Wouters PFJ, Proost P, Broekaert WF, Cammue BPA (2004) Processing in Arabidopsis thaliana of a heterologous polyprotein resulting in differential targeting of the individual plant defensins. Plant Sci 166:113–121
Ghag SB, Shekhawat UKS, Ganapathi TR (2012) Petunia floral defensins with unique prodomains as novel candidates for development of Fusarium wilt resistance in transgenic banana plants. PLoS ONE 7:e39557
Ghosh G, Purohit A, Ganguly S, Chaudhuri RK, Chakraborti D (2014) In-vitro shoot grafting on rootstock: an effective tool for Agrobacterium-mediated transformation of pigeon pea (Cajanus cajan). Plant Biotechnol 31:301–308
Ghosh G, Ganguly S, Purohit A, Chaudhuri RK, Das S, Chakraborti D (2017) Transgenic pigeonpea events expressing Cry1Ac and Cry2Aa exhibit resistance to Helicoverpa armigera. Plant Cell Rep 36(7):1037–1051
Guler L, Şevik M, Hasöksüz M (2014) Phylogenetic analysis of peste des petits ruminants virus from outbreaks in Turkey during 2008–2012. Turk J Biol 38:671–678
Horvath H, Rostoks N, Brueggeman R, Steffenson B, von Wettstein D, Kleinhofs A (2003) Genetically engineered stem rust resistance in barley using the Rpg1 gene. Proc Natl Acad Sci USA 100:364–369
Jayaraj J, Punja ZK (2007) Combined expression of chitinase and lipid transfer protein genes in transgenic carrot plants enhances resistance to foliar fungal pathogens. Plant Cell Rep 26:1539–1546
Jhoshi PK, Parthasarathy Rao V, Gowda CLL, Jones RB, Silim SN, Saxena KB, Kumar J (2001) The world chickpea and pigeon pea economies, facts trends, and outlook. ICRISAT, Patancheru, Andhra Pradesh, India. http://www.icrisat.org/PDF/Outlook%20rep-The%20World%20Chickpea.pdf.
Kaur A et al (2016) Pod borer resistant transgenic pigeon pea (Cajanus cajan L.) expressing cry1Ac transgene generated through simplified Agrobacterium-transformation of pricked embryo axes. Plant Cell Tissue Organ Cult 127:717–727
Khanna HK, Raina SK (2002) Elite indica transgenic rice plants expressing modified Cry1Ac endotoxin of Bacillus thuringiensis show enhanced resistance to yellow stem borer (Scirpophaga incertulas). Transgenic Res 11(4):411–423
Kranthi S, Kranthi RK (2004) Annual progress report: Baseline susceptibility of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) to Cry1Ac. Central Institute for Cotton Research, Nagpur, India. http://www.moef.gov.in/sites/default/files/geac/cicr0304.html
Krishna G et al (2011) Agrobacterium-mediated genetic transformation of pigeon pea [Cajanus cajan (L.) Millsp.] for resistance to legume pod borer Helicoverpa armigera. J Crop Sci Biotech 14:197–204
Lacerda AF, Vasconcelo EAR, Pelegrini PB, Grossi de Sa MF (2014) Antifungal defensins and their role in plant defense. Front Microbiol 5:116
Lacey AL, Kaya KH (2000) Field manual of techniques in invertebrate pathology Application and evaluation of pathogens for control of insect and other invertebrate pests. Springer Science & Business Media, Berlin, pp 571–575. https://doi.org/10.1007/978-94-017-1547-8
Lentini Z, Lozano I, Tabares E, Fory L, Domı’nguez J, Cuervo M, Calvert L (2003) Expression and inheritance of hypersensitive resistance to rice hoja blanca virus mediated by the viral nucleocapsid protein gene in transgenic rice. Theor Appl Genet 106:1018–1026
Li Z, Zhou M, Zhang Z, Ren L, Du L, Zhang B, Xu H, Xin Z (2011) Expression of a radish defensin in transgenic wheat confers increased resistance to Fusarium graminearum and Rhizoctonia cerealis. Funct Integr Genomics 11:63–70
Meiyalaghan S, Barrell PJ, Jacobs JME, Conner AJ (2011) Regeneration of multiple shoots from transgenic potato events facilitates the recovery of phenotypically normal lines: assessing a cry9Aa2 gene conferring insect resistance. BMC Biotechnol 11:93
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Murray HG, Thompson WF (1980) Rapid isolation of high molecular weight DNA. Nucl Acids Res 8:4321–4325
Nalluri N, Karri VR (2019) In vitro regeneration of ICP 8863 pigeon pea (Cajanus cajan (L.) Millsp.) variety using leaf petiole and cotyledonary node explants and assessment of their genetic stability by RAPD ANalysis. Indian J Sci Tech 12(9):1–10. https://doi.org/10.17485/ijst/2019/v12i9/140847
Nene YL, Sheila VK (1990) Pigeon pea: Geography and Importance. In: Nene YL, Hall SD, Sheila VK (eds) The Pigeon pea. CAB International, Wallingford, pp 1–14
Olli S, Guruprasad L, Kirti PB (2007) Characterization of defensin (Tfgd2) from Trigonella foenum-graecum. Curr Sci 93:365–369
Parashina EV, Serdobinskii LA, Kalle EG, Lavrova NV, Avetisov VA, Lunin VG et al (2000) Genetic engineering of oilseed rape and tomato plants expressing a radish defensin gene. Russian J Plant Physiol 47:417–423
Ramdas VR, Gaurav AD, Jaysing CR, Arun GI (2015) Rapid and highly competent shoot regeneration of pigeon pea (Cajanus cajan) using variable explants by in-vitro culture system. J Pharmacogn Phytochem 4:1–5
Ramu SV et al (2012) Expression of a synthetic cry1AcF gene in transgenic pigeon pea confers resistance to Helicoverpa armigera. J Appl Entomol 136:675–687
Rana N, Ganguli J, Agale SV (2017) Screening of Pigeon pea genotypes under field conditions against pod borer complex. J Entonol Zool Studies 5:1914–1920
Ranga Rao GV, Shanower TG (1999) Identification and management of pigeonpea and chickpea insect pests in asia. Information bulletin no. 57. (In En. Summaries in En, Fr.) Patancheru, 502 324, A.P., India: International crops research institute for the semiarid tropics. ISBN 92–9066–412–6
Rao SK, Sreevathsa R, Sharma PD, Keshamma E, Kumar MU (2008) In-planta transformation of pigeon pea: A method to overcome recalcitrance of the crop to regeneration in-vitro. Physiol Mol Biol Plants 14:321–328
Reed W, Lateef SS (1990) Pest management. In: Nene YL, Hall SD, Sheila VK (eds) The Pigeon pea. CAB International, Wallingford, UK, pp 349–374
Sarkar S, Roy S, Ghosh SK, Basu A (2019) Application of lateral branching to overcome the recalcitrance of in-vitro regeneration of Agrobacterium-infected pigeon pea (Cajanus cajan (L.) Millsp.). Plant Cell Tissue Organ Cult 137:23–32
Sarkar S, Roy S, Ghosh SK (2021) Development of marker-free transgenic pigeon pea (Cajanus cajan) expressing a pod borer insecticidal protein. Sci Rep 11(1):1–16
Sharma KK, Lavanya M, Anjaiah V (2006) Agrobacterium mediated production of transgenic pigeon pea (Cajanus cajan L. Millsp.) expressing the synthetic Bt cry1AB gene. In Vitro Cell Dev Biol Plant 42:165–173
Shin R, Han JH, Lee GJ, Paek KH (2002) The potential use of a viral coat protein gene as a transgene screening marker and multiple virus resistance of pepper plants coexpressing coat proteins of cucumber mosaic virus and tomato mosaic virus. Transgenic Res 11:215–219
Singh ND, Sahoo L, Saini R, Sarin NB, Jaiwal PK (2002) In-vitro shoot organogenesis and plant regeneration from the cotyledonary node and leaf explants of pigeon pea (Cajanus cajan). Physiol Mol Biol Plants 8:133–140
Singh S, Kumar NR, Maniraj R et al (2018) Expression of Cry2Aa, a Bacillus thuringiensis insecticidal protein in transgenic pigeon pea confers resistance to gram pod borer Helicoverpa armigera. Sci Rep 8:8820. https://doi.org/10.1038/s41598-018-26358-9
Sinha KS (1977)Food legumes: Distribution, adaptability, and biology of yield, In FAO Plant Production and Protection Paper 3:1–102. https://www.cabdirect.org/cabdirect/abstract/19786724643.
Srivastava J (2013) In-vitro regeneration protocol for pigeon pea - a review. Int J Food Agric Vet Sci 3:63–76
Surekha C et al (2005) Agrobacterium-mediated genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) using embryonal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci 169:1074–1080
Surekha Ch, Arundhati A, Rao S (2007) Differential response of Cajanus cajan varieties to transformation different strains of Agrobacterium. J Biol Sci 7:176–181
Tailor RH, Acland DP, Attenborough S, Cammue BP, Evans IJ, Osborn RW, Ray JA, Rees SB, Broekaert WF (1997) A novel family of cystiene-rich antimicrobial peptides from seeds of Impatiens balsamina is derived from a single precursor protein. J Biol Chem 272:24480–24487
Tamiru A, Khan RZ, Bruce AJT (2015) New directions for improving crop resistance to insects by breeding for egg induced defense. Curr Opin Insect Sci 9:51–55
Terras FR, Goderis IJ, Van Leuven F, Vanderleyden J, Cammue BPA, Broekaert WF (1992a) In vitro antifungal activity of a radish (Raphanus sativus L.) seed protein homologous to nonspecific lipid transfer proteins. Plant Physiol 100:1055–1058
Terras FR, Schoofs H, De Bolle MFC, Van Leuven F, Rees SB et al (1992b) Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J Biol Chem 267:15301–15309
Terras FR, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, van Leuven F, Vanderleyden J (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Thomma BP, Cammue BP, Thevissen K (2002) Plant defensins. Planta 216:193–202
Tripathi AK, Prajapati V, Aggarwal KK, Kumar S (2001) Toxicity, feeding deterrence, and effect of activity of 1, 8-cineole from Artemisia annua on progeny production of Tribolium castanaeum (Coleoptera: Tenebrionidae). J Eco Entomol 94:979–983
Karri V, Pulugurtha Bharadwaja K (2013) Tandem combination of Trigonella foenum-graecum defensin (Tfgd2) and Raphanus sativus antifungal protein (RsAFP2) generates a more potent antifungal protein. Funct Integr Genom 13(4):435–443
Vasavirama K, Kirti PB (2012) Increased resistance to late leaf spot disease in transgenic eanut using a combination of PR genes. Functional Integrated Genomics 12:625–634. https://doi.org/10.1007/s10142-012-0298-8
Vasavirama K, Kirti PB (2013b) Constitutive expression of a fusion gene comprising Trigonella foenum-graecum defensin (Tfgd2) and Raphanus sativus antifungal protein (RsAFP2) confers enhanced disease and insect resistance in transgenic tobacco. Plant Cell Tissue Organ Culture 115:309–319. https://doi.org/10.1007/s11240-013-0363-6
Vijayan S, Singh NK, Shukla P, Kirti PB (2013) Defensin (TvD1) from Tephrosia villosa exhibited strong anti-insect and antifungal activities in transgenic tobacco plants. J Pest Sci. https://doi.org/10.1007/s10340-012-0467-5
Vishwadhar SK et al (2008) Forecasting of Helicoverpa armigera infestation on long duration pigeon pea in central Uttar Pradesh. J Food Leg 21:189–192
Yadav A, Kumar A, Yadav R, Misra JP, Kumar R (2016) In-vitro regeneration through organogenesis in pigeon pea (Cajanus cajan). J Cell Tissue Res 16:5485–5490
Zhu JQ, Liu S, Ma Y, Zhang JQ, Qi HS, Wei ZJ, Yao Q, Zhang WQ, Li S (2012) Improvement of pest resistance in transgenic tobacco plants expressing dsRNA of an insect-associated gene EcR. PLoS ONE 7(6):e38572
Acknowledgements
The authors are grateful to Science & Engineering Research Board (SERB)–Young Scientist Scheme (YSS) (SB/YS/LS-83/2014 to Dr. K. Vasavi Rama) for their encouragement and financial support to this project. The authors are thankful to Prof. P. B. Kirti, Department of Plant Sciences, University of Hyderabad for his continuous guidance and valuable support to complete this work. The authors also acknowledge the support of Department of Biotechnology, GITAM School of Technology and Department of Biotechnology, GITAM School of Science, GITAM (Deemed to be University), Visakhapatnam in successful completion of this study.
Funding
This study was financially endorsed by grants from Science and Engineering Research Board (SERB), Department of Science & Technology, Government of India (SERB-YSS (SB/YS/LS-83/2014 to Dr. K. Vasavi Rama).
Author information
Authors and Affiliations
Contributions
VK designed and guided NN to work on this specific topic to acquire data. NN prepared manuscript according to guidelines under the supervision of VK. Finally the manuscript was checked and corrected by VK for submission in favor of publication. Both worked hard in the analysis of data to complete this investigation. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nalluri, N., Karri, V. Over-expression of Trigonella foenum-graecum defensin (Tfgd2) and Raphanus sativus antifungal protein (RsAFP2) in transgenic pigeonpea confers resistance to the Helicoverpa armigera. Plant Cell Tiss Organ Cult 152, 569–582 (2023). https://doi.org/10.1007/s11240-022-02431-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02431-0