Abstract
Rumex thyrsiflorus Fingerh. is a dioecious species with heteromorphic sex chromosomes. Due to sexual dimorphism and the content of bioactive constituents used in pharmacology, this species is an interesting object of study. A complex analysis of selected physiological and biochemical aspects of the sex-related response to heat stress in vitro and in vivo was carried out. The experiment included in vitro regenerated plants and plants obtained from seeds. Regenerants were obtained from hypocotyls on Murashige and Skoog (MS) medium supplemented with 2.27 μM thidiazuron (TDZ). The sex of the plants was determined by molecular analysis based on genetic sex markers. Analysis of the main photosynthetic parameters indicated that in vitro regenerated plants showed a decrease in photosystem II (PSII) activity when directly exposed to a stressor. However, in contrast to the seed-derived plants, they adapted efficiently to the recovery conditions within 1 week after the stress was terminated. Furthermore, in vitro regenerated male and female plants acclimatised well to field conditions and showed greater stress tolerance based on better efficiency of the photosynthetic apparatus and the highest chlorophyll a/b ratio. In case of plants derived from seeds, male plants were less sensitive to heat stress and showed greater stability of PSII at high temperatures compared to female plants. The results showed that the response to high-temperature stress depends on the sex and the origin of the plants, i. e. in vitro regenerated plants versus plants obtained from seeds. Even short-term heat stress resulted in differences in photosynthetic efficiency. Biochemical analysis of antioxidant activity in response to heat stress, carried out for the first time in Rumex thyrsiflorus, has allowed the identification of the following forms of superoxide dismutase (SOD): manganese (MnSOD), iron (FeSOD) and two copper-zinc isoforms (Cu/ZnSOD I and Cu/ZnSOD II).
Key message
The response to heat stress depends on the sex and origin of the plants (regenerated in vitro versus obtained from seeds). Three forms of superoxide dismutase were identified for the first time in Rumex thyrsiflorus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rumex thyrsiflorus Fingerh. (thyrse sorrel) is a dioecious species of the genus Rumex, family Polygonaceae Juss. Individuals of R. thyrsiflorus are characterised by a “Drosophila-type” sex determination, in which sex is determined by the ratio of sex chromosomes to autosomes (X:A) (Mariotti et al. 2009). In female plants, the karyotype consists of the following chromosomes: 2n = 12A + XX, and in male plants: 2n = 12A + XY1Y2 (Żuk 1963). Although, it serves as a model object for studies on chromosomes, sex chromatin and sex ratio (Błocka-Wandas et al. 2007; Grabowska-Joachimiak et al. 2012), thyrse sorrel is also known as a source of biologically active compounds of pharmaceutical importance, such as flavonoids, phenolic acids, and anthraquinones (Orbán-Gyapai et al. 2017).
Most plant species are hermaphroditic and only about 6% of angiosperms are dioecious, i.e. there are individuals with separate sexes (Renner 2014). Although in the case of chromosomal sex determination the primary sex ratio should be 1:1 (Korpelainen 2002), biased sex ratios can be observed in populations of many dioecious plants. Field et al. (2013) reported considerable sex ratio heterogeneity in dioecious flowering plants. Most of this variation appears to reflect the demographic characteristics of the populations and the differential response of the sexes to environmental stress. They found that growth and survival rates of females were lower under more stressful conditions. However, experiments with Rumex thyrsiflorus by Ślesak et al. (2017) showed that there is no competition between the sexes during seed germination (germination time and seedling length) when grown under uniform growing conditions. Dimorphic species do not always show differences in stress tolerance between the sexes; and when sex differences do occur, they appear to be very species-specific, with stress tolerance being greater in females than in males in some species and the opposite in others. The causes of such sex-specific differences are not yet well understood, and further physiological studies and comparative analyses are needed.
Predicting the effects of global warming on dioecious plants is a challenge for current research. Global average temperatures are likely to increase by up to 3.9 °C by the end of this century (Munné-Bosch 2015). In addition, climate change factors such as the projected temperature increase and the availability of CO2 and O3 will have a profound impact on oxidative signalling in plants, particularly in relation to photosynthetic metabolism and the response to environmental stress. Studying the mechanisms of stress adaptation in male and female dioecious plants is important to understand the evolution of these species in the context of global warming (Juvany and Munné-Bosch 2015).
Temperature is known to affect plant growth, productivity, physiology, and biochemistry (Zhang et al. 2018). Rising temperatures cause severe damage to protein complexes, especially the photosystem II (PSII), which is a component of the photosynthetic electron transport chain (Camejo et al. 2005). There is much evidence to suggest that a common feature of most stresses in plants is an imbalance between pro- and antioxidant responses in the plant cell, manifesting as oxidative stress. Stress responses are very often associated with increased production of reactive oxygen species (ROS) such as superoxide anion radicals (O2 –•), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH), which lead to oxidative stress in plant cells. ROS levels can increase due to various environmental factors such as light, drought or temperature stress. An excess of ROS, which causes oxidative stress is harmful to cells, as these molecules can damage nucleic acids, proteins and lipids (Ślesak et al. 2006; Szymańska et al. 2017).
Plants, like other organisms, have developed a mechanism to effectively eliminate ROS called the antioxidant system. In this system, two types of antioxidants can be distinguished: non-enzymatic and enzymatic. Enzymes that remove ROS include mainly superoxide dismutases (SOD), catalases (CAT), and various peroxidases (POX). The role of antioxidants is to regulate ROS homeostasis and reduce oxidative damage in plant cells (Zhang et al. 2010; Havaux and Niyogi 1999; Szymańska et al. 2017). In oxidative stress, the synchronised action of the antioxidant system leads to the elimination of ROS and protects plants from oxidative damage. Depending on the intensity of the stress and its duration, the content of synthesised antioxidants may vary. The antioxidant response also depends on the stress tolerance of the plants and often varies in different species. Photosynthesis, for example, is one of the processes most sensitive to temperature stress, which can damage PSII localised in the thylakoid membrane. Dysfunction of PSII leads to a decrease in photosynthetic efficiency (Zhai et al. 2020). When stress is extreme, photosynthesis is inhibited within minutes. Heat stress affects the water status in leaves and the intracellular CO2 concentration as a result of stomata closure. All these reactions lead to a decrease in photosynthetic rate (Szymańska et al. 2017).
Tissue culture makes it possible to grow plants under controlled conditions, independent of seasons and changing climate. This technique is used as a method for stress assessment by managing large populations in a defined space (Pérez-Jiménez and Pérez-Tornero 2020). The procedure for micropropagation of R. thyrsiflorus was developed (Ślesak et al. 2017). Molecular analysis based on the identification of the sex of explants using genetic sex markers makes it possible to maintain the culture of female or male plants as a model, e.g. for the study of sex-related production of secondary metabolites or sex-related stress tolerance. Studies on the sex of Rumex thyrsiflorus seeds (Kwolek and Joachimiak 2011) and adult individuals (Korpelainen 2002) revealed a female-biased sex ratio. Our previous studies have shown that female R. thyrsiflorus plants regenerated in vitro are a more valuable source of phenolic acids, flavonoids or catechins compared to males. This could be the result of a sex-specific response to stressful in vitro conditions, where plants are exposed to stress factors such as specific environmental conditions inside culture vessels e. g. high humidity and low gas exchange (Pospíšilová et al. 1999). The aim of the present studies was therefore to test the general hypothesis that the response of R. thyrsiflorus to high temperature stress depends on the sex of the plant and its origin, including previous growing conditions, i.e. in vitro regenerated plants versus plants obtained from seeds. The hypothesis was tested using selected stress-related biochemical and physiological analyses.
Materials and methods
Plant material and culture conditions
Seeds of Rumex thyrsiflorus Fingerh. (POLAN, Poland) were sterilized in 70% (v/v) EtOH for 1 min., then in a 50% (v/v) commercial bleach (< 5% sodium hypochlorite) for 12 min. The seeds were then rinsed 3 times in sterile distilled water. Seeds were germinated for 11 days in a culture room at 25° ± 3 °C, 16 h/8 h photoperiod (day/night), and fluorescent white light (60-90 μmol m−2 s−1). Hypocotyls (ca 5 mm) were isolated and placed on MS medium (Murashige and Skoog 1962) supplemented with 3% (w/v) sucrose, 2.27 μM thidiazuron (TDZ) and 0.8% (w/v) agar, as described by Ślesak et al. (2017). Five explants were inoculated onto a Petri dish (60 mm). The experiment was repeated 3 times (total 375 explants/75 Petri dishes). Passage of cultures into fresh medium was done every 3-4 weeks. Adventitious shoots regenerated from the morphogenic callus were rooted on ½ MS medium supplemented with 2% (w/v) sucrose, 0.8% (w/v) agar, and 2.46 μM indole–3–butyric acid (IBA). After 2-7 weeks, the regenerants that had developed a root system were transferred to pots with soil and acclimated to the ex vitro conditions in the culture room (conditions as described above).
For the control plants, the seeds of R. thyrsiflorus were placed on Petri dishes with moist filter paper (non-sterile) to initiate seed germination. After 7 days, the obtained seedlings were transferred to pots with soil. The control plants were grown in the culture room under the same conditions as the acclimatised regenerants (see above).
Identification of the sex and analysis of the sex ratio
To determine the sex of the plants used for the experiment, the following plant material was used: (1) leaves from control plants, (2) cotyledons and roots excised from 11-day-old seedlings, the same from which the hypocotyls were also used as explants in the in vitro micropropagation.
DNA was extracted using the hexadecyltrimethylammonium bromide (CTAB) method (Gawel and Jarret 1991) with modifications (Kwolek and Joachimiak 2011). A PCR-based technique using DNA markers on the Y chromosomes was used to identify the sex of the plants. Primers designed by Korpelainen (2002), that amplify the male-specific RAYSI sequence on the Y chromosomes of R. acetosa and its close relatives (Navajas-Pérez et al. 2006) were used: RAY-F (5′-ACTCGAATGTAAGCATTTGGTGTCCTCCATAAAGTGGA-3′) and RAY-R (5′-ACTACACGATTGTCCATAAAGTGGA-3′). In addition, primers URG08-F (5′-CCAATTGGTCTCAACTAGAACA-3′) and URG08-R (5′-TGTTATAGGTTGGACTGCCA-3′) which amplify the male-specific repetitive sequence RAYSII in R. acetosa L. were used (Mariotti et al. 2009).
To check the quality of the template DNA, primers amplifying the autosomal sequence RAE730 were used: R730-A (5′-CTCGGACCAATT ATCTCAT-3′) and R730-B (5′-CATTATTTGGGAGCC GAT-3′) (Navajas-Pérez et al. 2005). The amplification reaction was performed in a T100 Thermal Cycler (BioRad) (for a description of the reaction mixtures and programmes see Ślesak et al. 2015).
PCR products were separated in 1% (w/v) agarose gel in 1×TBE buffer with Simply Safe (EURx) or Syngen GreenDNA Gel Stain (Syngen Biotech) for 60 min at a constant voltage of 120 V. Perfect Plus 100 bp DNA Ladder (EURx) or GeneRuler 100 bp DNA Ladder (Thermo Scientific) were used as the molecular standards.
The ratio of male to female individuals was estimated according to Rychlewski and Zarzycki (1975): M:F = 1:F/M, where F-female plants, M-male plants.
Short-term heat stress experiment
For the experiment on the influence of short-term high temperature stress, in vitro regenerated plants and control plants germinated from seeds (of the same age, about 11 weeks-old) were placed in a phytotron chamber (Biogenet) at a light intensity of 190 µmol m−2 s−1, photoperiod 16 h/8 h (day/night). The experiment consists of four stages (I-IV) (Fig. 1). In the first stage (I) the plants were grown at 25 °C (7 days), in the second stage (II) the plants were exposed to heat stress (temperature 35 °C) for 7 days, in the third stage (III) the plants were again placed at 25 °C, as in the I stage (recovery conditions), and in the fourth stage (IV) the plants were acclimated to field conditions (Gronostajowa 3, Kraków, Poland, 50°01′39″N, 19°54′12″E). At each stage the following analyses were carried out: measurements of chlorophyll a (Chl a) fluorescence of PSII, photosynthetic pigment content and determination of antioxidant enzymes, i.e. SOD and CAT activities (for details see Fig. 1).
Scheme of the experiment on the influence of short-term heat stress on Rumex thyrsiflorus. In vitro regenerated plants and control plants (obtained from seeds) were placed in a phytotron chamber; I-first stage of the experiment, the plants grew in the phytotron chamber at a temperature of 25 °C; II-second stage, the plants from stage I were placed in a high temperature of 35 °C, III-third stage, the plants from stage II were again placed in a temperature of 25 °C (recovery conditions), IV-fourth stage, the plants from stage III were placed in field conditions. After 7 days in stage I-III and after 9 weeks in stage IV the fluorescence of chlorophyll a was measured and the activity of antioxidant enzymes (SOD, CAT) and the content of photosynthetic pigments were determined; FR female regenerants, MR male regenerants, FC female control, MC male control
Measurements of chlorophyll a fluorescence
Photosynthetic efficiency based on Chl a fluorescence of PSII was measured at each experimental stage (Fig. 1) using a HandyPEA portable fluorimeter (Hansatech, UK). Chl a fluorescence induction curves were determined on dark adapted leaves (20 min.). The following parameters were analyzed: maximum quantum yield of PSII photochemistry in the dark-adapted state (Fv/Fm = (Fm − F0)/Fm), minimal (F0), maximal (Fm) and variable fluorescence (Fv), overall performance index of PSII (PI) and time to maximal fluorescence (TFm). Each time, after measuring chlorophyll fluorescence, two leaves were taken from each individual, frozen in liquid nitrogen and stored in the deep freezer for further analysis.
Analysis of the photosynthetic pigment content
Photosynthetic pigment content was analysed in lyophyllised (Labconco Freezone 77530–32, A. G. A. Analytical) leaves of three randomly selected male and female regenerants and control plants at each stage of the experiment. The analysis was performed according to Lichtenthaler and Buschmann (2001) with some modifications. Plant tissue was homogenised in 100% acetone cooled to – 20 °C and small amount of MgCO3. After centrifugation at 4000 rpm at 4 ºC for 5 min, the supernatant was diluted 1:4 in cuvettes (200 μl extract + 800 μl 100% acetone). Absorbance was measured using a spectrophotometer (SmartSpec Plus, BioRad) 3 times at the following wavelengths: 662, 645 and 470 nm. The content of Chl a, chlorophyll b (Chl b) and carotenoids (car) was calculated using the equations of Lichtenthaler (1987) and expressed on a dry weight basis (mg g−1 DW). The sum of Chl a and Chl b was defined as total chlorophyll content, according to Lichtenthaler (1987).
Analysis of the activity of selected antioxidant enzymes (SOD, CAT)
Preparation of soluble proteins (crude extract) from plants
Five randomly selected leaves (pooled samples) from male and female individuals of both regenerants and control plants were used for analysis at each stage of the experiment. The material 1 or 1.5 g FW was mixed in a ratio of 1:3 [g fresh weight: ml homogenisation buffer (100 mM Tricine, 3 mM MgSO4 · 7H2O, 1 mM DTT, 3 mM EGTA, pH 8.0)] (Miszalski et al. 1998 with modifications).
The next step was to centrifuge the extract for 5 min at 4000 rpm at 4 °C. To determine the concentration of soluble proteins, the Bradford method (1976) was chosen, using the Thermo Fisher Scientific protein assay (USA). Bovine serum albumin (BSA) (Merck) was used as a protein standard.
Native PAGE
Native polyacrylamide gel electrophoresis (PAGE) was performed as previously described by Miszalski et al. (1998). After protein determination, samples were separated in a 12% polyacrylamide gel for SOD activity staining analysis or with modification in a 10% polyacrylamide gel for CAT activity staining at 4 °C and 180 V. The polyacrylamide gels were scanned using an EPSON Perfection V600 Photo scanner.
SOD activity
SOD activity in the gels was visualised by activity staining according to Beauchamp and Fridovich (1971). For determination of SOD activity, gels were incubated at room temperature in the dark on a shaker for about 20 min in standard staining phosphate buffer (50 mM, pH 7.8) containing 1 mM EDTA, 2.8 mM TEMED, 22 μM riboflavin and 245 μM NBT. The gels were then exposed to white light on a transilluminator (UVP) until the SOD activity bands became visible.
To inhibit Cu/ZnSOD activity, the gels were incubated in buffer containing 3 mM KCN. To inhibit Cu/ZnSOD and FeSOD activity, H2O2 was added to the staining buffer to a final concentration of 5 mM.10 mM 3-amino-1,2,4-triazole was also added to the standard staining buffer to eliminate bands indicating the presence of catalase (CAT).
Staining of CAT activity
CAT activity was determined according to the procedure described by Woodbury et al. (1971). For CAT detection, the gels were washed 3 times in distilled water for 10 min. and then incubated in H2O2 solution (0.03% v/v) for 10 min. They were then washed in distilled water and stained for 10 min in a 1:1 mixture of 2% (w/v) FeCl3 and 2% (w/v) K3Fe(CN)6.
Data analysis
Statistics for all results concerning PSII activity of plants used for the short—term heat stress experiment: in vitro regenerated plants and plants obtained from seeds are reported as mean ± SE (standard error). The Kruskal–Wallis test and Dunn’s Multiple Comparison Test as post-test were used to verify the statistically significant differences in the values of the following parameters: TFm, F0, Fm, Fv, Fv/Fm, PI. The analysis was done for (1) female (FR) and male (MR) regenerants and female (FC) and male (MC) control plants between four stages (I–IV) of the experiment and (2) within each stage all the four mean values were compared (FR vs FC, MR vs MC, FR vs MR, FC vs MC). Statistically significant differences were verified for p < 0.05 (*), p < 0.001 (**), p < 0.0001 (***). Statistical analysis was performed using GraphPad Prism software (v. 5.03).
Results
Plants used for the short-term heat stress experiment: in vitro regenerated plants and control plants obtained from seeds
Induction of callus tissue was observed on most hypocotyls 2 weeks after the start of culture on MS medium supplied with 2.27 μM TDZ. Callogenesis was observed on the entire surface of the explants 4 weeks after culture induction (Fig. 2a, b). Indirect morhogenesis (via callus) was observed. The formation of adventitious shoots was noted 7 days after callus induction (Fig. 2c, d). The regenerated shoots were isolated and inoculated on rooting medium ½ MS supplemented with 2% sucrose and 2.46 μM IBA. Root formation was observed after 1-2 weeks (Fig. 2e). The rooted plantlets (79 individuals) were acclimated to the ex vitro conditions with an efficiency of 79.5% (63 individuals). In the case of the control plants germinated from seeds, 54 plants were obtained. The in vitro regenerated plants and the control plants were placed in the phytotron chamber for the duration of the I, II and III stages of the short-term heat stress experiment (Fig. 2f). At the IV stage, they were successfully acclimated to field conditions (Fig. 2g).
In vitro regenerated plants and control plants obtained from seeds used for the short-term heat stress experiment. Micropropagation in vitro on MS + 2.27 μM TDZ a-e; callus induction on hypocotyl surface (4 weeks culture) a, b; regeneration of adventitious shoots (8 weeks culture) c, d; isolated adventitious shoots were inoculated on rooting medium (½ MS + 2% sucrose + 2.46 μM IBA) and root formation was observed after 1-2 weeks e; regenerants and control plants were placed in the phytotron chamber for the duration of the I, II and III stage of experiment f and acclimated to field conditions (IV stage of the experiment) g. Bars 5 mm a-d, 2 cm e, 7 cm f, 8 cm g
Molecular analysis
For the male individuals, a PCR reaction with the primers RAY-R and RAY-F, which amplify RAYSI sequences, gave a product of about 930 bp (Fig. 3a). In the case of primers URG08-R and URG08-F, which amplify the RAYSII sequence, two products of about 700 and 600 bp were found in male individuals (Fig. 3b). Amplification of the autosomal RAE 730 sequence with primers R730-A and R730-B confirmed the good quality of the template DNA (Fig. 3c).
The ratio of male to female individuals in the control and in vitro regenerated plants was M:F = 1:3.5 and M:F = 1:2.5, respectively.
Measurement of PSII activity
The mean values of the following photosynthetic activity parameters based on Chl a fluorescence: F0, Fm, Fv, Fv/Fm, TFm, and PI at each stage of the experiment were compared.
PSII efficiency within the experimental stages
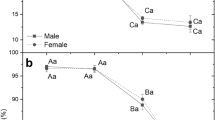
The comparative analysis of the mean values of the studied parameters of photosynthetic activity within the different stages of the experiment showed statistically significant differences between FR and FC plants only in the following cases: (1) F0 in each stage of the experiment (Fig. 4a–d). At the I, II, and III stage, the mean value of F0 was higher in the in vitro regenerated female plants than in the control plants (Fig. 4a–c). In contrast, at the IV stage (acclimation to ex vitro conditions), the mean value of F0 was higher in FC plants than in FR (Fig. 4d); (2) the mean values of Fm at the III stage of the experiment (Fig. 4e) were lower in FC plants than in FR; (3) Fv/Fm at the IV stage of the experiment (Fig. 4f). In this case, highly statistically significant differences (p < 0.0001) were observed between FC plants, with the mean value of this parameter being significantly lower compared to FR (Fig. 4f); (4) plant performance index (PI) at the I stage of the experiment was higher in FC plants than in FR (Fig. 4g).
The mean value of minimum Chl a fluorescence yield in the dark-adapted state (F0) at stages I a, II b, III c, IV d of the experiment; the mean value of the maximum Chl a fluorescence yield in the dark-adapted state (Fm) at the III stage of the experiment e, maximum quantum yield of PSII photochemistry (Fv/Fm) at the IV stage of the experiment f, plant performance index (PI) at the I stage of the experiment g, expressed in arbitrary units [a. u.]; FR female regenerants, FC female control, MR male regenerants, MC male control; data are mean ± SE (n = 11–44). Asterisks indicate significant differences between means at the level of p < 0.05 (*), p < 0.001 (**), p < 0.0001 (***) according to Dunn’s Multiple Comparison Tests
PSII efficiency – comparison between the stages of the experiment
The comparative analysis of the mean values of the studied parameters between the four stages of the experiment showed highly statistically significant differences (p < 0.0001) in the following parameters: F0, Fm, Fv, Fv/Fm, TFm and PI (Figs. 5, 6).
Comparison of the mean value of the minimum Chl a fluorescence yield in the dark-adapted state (F0) a, the maximum Chl a fluorescence yield in the dark-adapted state (Fm) b and maximum variable fluorescence (Fv) c between the I-IV stages of the experiment expressed in arbitrary units [a. u.]; FR female regenerants, FC female control, MR male regenerants, MC male control; data are mean ± SE (n = 11-44). Statistically significant differences were verified for p < 0.05 (*), p < 0.001 (**), p < 0.0001 (***) according to Dunn’s Multiple Comparison Tests
Comparison of the mean value of the maximum quantum yield of PSII photochemistry (Fv/Fm) a, the time at which the maximum fluorescence value (Fm) was reached (TFm) b and the plant performance index (PI) c between the I-IV stages of the experiment expressed in arbitrary units [a. u.]; FR female regenerants, FC female control, MR male regenerants, MC male control; data are mean ± SE (n = 11-44). Statistically significant differences were verified for p < 0.05 (*), p < 0.001 (**), p < 0.0001 (***) according to Dunn’s Multiple Comparison Tests
The comparison of the mean value of the minimum Chl a fluorescence yield in the dark-adapted state (F0) showed statistically significant differences in female control plants between I and IV, as well as III and IV stage of experiment (Fig. 5a). Moreover, only FR showed a lower mean value of F0 during acclimation to field conditions, compared to the other groups analysed. Analysis of the mean value of the maximum Chl a fluorescence yield in the dark-adapted state (Fm) revealed differences between the I and IV stages in the case of FC and FR (Fig. 5b). In both cases, the value was lower at the IV stage of the experiment. A similar trend was observed for the maximum variable fluorescence (Fv), additionally the mean value of this parameter was significantly lower in the FC plants at the II stage of the experiment compared to the stage I (Fig. 5c).
Comparative analysis of the mean value of the maximum quantum yield of PSII photochemistry (Fv/Fm) showed significant differences between the I and IV stages of the experiment in both MC and FC plants. In this case, the mean value of this parameter was lower during acclimation to field conditions (IV stage) (Fig. 6a). Moreover, the differences (decrease in Fv/Fm value) were visible between I and II, as well as III and IV stage of experiment in FC plants. In case of the time at which the maximum fluorescence value was reached (TFm) (Fig. 6b) FR and FC plants showed statistically significant differences between all experimental stages (I and IV, II and IV, III and IV), and MR and MC plants only between II and IV stages. In all cases, the value of this parameter was highest at the II stage (short-term heat stress) and lowest at the IV stage (field conditions).
A similar trend, in terms of statistically significant differences, was observed in the analysis of the plant performance index (PI) (Fig. 6c). In general, a decrease in the value of this parameter was observed after treatment with high temperatures and an increase in plants that grew for several weeks under field conditions (IV stage).
Analysis of the content of photosynthetic pigments
Chlorophylls (a and b) were most abundant in the leaves of the plants in the first three stages of the experiment (Fig. 7). In the first stage of the experiment, the chlorophylls/carotenoids ratio was highest in the in vitro regenerated female plants (Fig. 7a). A general trend of decreasing carotenoid content was observed at the II and III stages, excluding male control plants at the III stage of the experiment. At the IV stage of the experiment (field conditions), carotenoids were the dominant pigments in the leaves of the tested plants, except for the MC individuals, where twice as many chlorophylls as carotenoids were found. Moreover, the highest mean content of carotenoid pigments was found in MR and FR at the IV stage (Fig. 7d). Similarly, the mean ratio of chlorophyll a/b at the IV stage of the experiment was highest in MR and FR (Fig. 8).
SOD and CAT activities
Manganese (MnSOD), iron (FeSOD), and two copper-zinc isoforms (Cu/ZnSOD I and Cu/ZnSOD II) were identified (Fig. 9). The presence of Cu/ZnSOD I, Cu/ZnSOD II and MnSOD was observed in control plants and in vitro regenerated plants of both sexes at all stages of the experiment. FeSOD was identified only in FC and MC plants at II stage (heat stress) of the experiment (Fig. 9b).
Native polyacrylamide gel electrophoresis (PAGE) of the different SOD forms in in vitro regenerated and control plants of Rumex thyrsiflorus. Inhibitors were KCN and H2O2: 20 μg protein was loaded per lane; stages of the experiment: I a, II b, III c, IV d; MC male control, FC female control, MR male regenerants, FR female regenerants
CAT activity was observed in leaves of in vitro regenerated plants and control plants of both sexes at all stages of the experiment (Fig. 10), except for male and female regenerants at the IV stage (field conditions) (Fig. 10d).
Discussion
The sex differences could be due to different resource management and are most evident under conditions unfavourable to the plant. Reproductive costs are much higher in females than in males. Thus, the investment in reproduction in females results in less energy that can be used for growth or defence, making females less resistant to stress (Liu et al. 2021). However, dimorphic species do not always show sex-specific differences in stress response. An example of such a species is Salix viminalis, where there were no differences between the sexes under flood stress (Zhai et al. 2020). An interesting species described in Juvany and Munné-Bosch (2015) is Honckenya peploides, which showed no sex differences in response to salt stress, but under water stress, females showed higher tolerance than males. In contrast, in Populus, male individuals were more tolerant to drought stress than females because they had higher values of total chlorophyll concentration, net photosynthetic rate and superoxide dismutase and peroxidase activities (Chen et al. 2014; Hao et al. 2020). In general, the responses of a given sex are highly species-specific and depend on the type of stress factor.
High temperature stress as well as many other abiotic stresses as a result of climate change, have become recently a serious threat to plants, affecting their growth and development. Throughout the life cycle, plants are exposed to temperature fluctuations, but globally elevated temperatures have a negative impact on plant biomass production. Nevertheless, plants have developed a molecular system that helps them to perceive changes and adapt to unfavourable conditions (Ahuja et al. 2010).
PSII efficiency within the experimental stages—comparison between sex and origin of the plants
The comparative analysis of the mean values of selected chlorophyll fluorescence parameters of R. thyrsiflorus within the different stages of the experiment showed significant differences only between FR and FC plants. One of the analysed parameters was the mean value of the minimum Chl a fluorescence yield in the dark-adapted state (F0). This parameter describes the level of fluorescence when primary quinone electron acceptors of PSII (QA) are maximally oxidised, i.e. the PSII centres are open. It indicates the loss of excitation energy during its transfer from the energy antennas to the PSII reaction centre (Baker and Rosenqvist 2004). Higher values of this parameter indicate lower efficiency of transfer of excitation energy between chlorophyll molecules. In the present experiment, the mean value of this parameter increased after the heat stress treatment at FR compared to FC plants, in contrast to the last stage of the experiment (IV), where the opposite results were obtained, indicating that the in vitro regenerated female plants adapted better to the field conditions. A similar trend was observed for the plant performance index (PI).
Analysis of the mean value of the maximum fluorescence level of dark adapted leaves (Fm), which indicates the fluorescence level when QA is maximally reduced (PSII centres are closed), revealed a statistically significant decrease at the III stage in FC plants (7 days after high temperature stress) compared to FR. Similar results were obtained for maximum quantum efficiency of PSII photochemistry (Fv/Fm) at IV stage, where a highly statistically significant decrease in mean Fv/Fm was observed in female control plants compared to female regenerants. The results described above could indicate a better efficiency of the photosynthetic apparatus in in vitro regenerated plants and an impairment of photosynthesis in seed-derived control plants.
Fv/Fm is used to detect stress-induced perturbations in the photosynthetic apparatus, as a decrease in Fv/Fm may be due to the development of slowly relaxing quenching processes and photodamage of PSII reaction centres, which reduce the maximum quantum efficiency of PSII photochemistry (Baker and Rosenqvist 2004). Under stress conditions, the value of the Fv/Fm parameter may decrease (Kalaji and Bosa 2011). As a result of high temperatures, a decrease in Fv/Fm was observed in wheat cultivars in a study by Sharma et al. (2015). However, Zhai et al. (2020) observed a relatively stable value of the parameter Fv/Fm under elevated temperature conditions in Salix viminalis. Fm is also related to the chlorophyll content of the tissue (Kalaji and Bosa 2011), which can be seen in the case of stage IV of the experiment, where the lowest Chl a + b content was recorded in most of the analysed leaves of R. thyrsiflorus compared to stage I, II and III.
No statistically significant differences were found in the values of the main photosynthetic parameters in male plants obtained from seeds, indicating a lower sensitivity to heat stress and a greater stability of the photosynthetic apparatus in response to high temperature stress compared to female plants obtained from seeds.
PSII efficiency-comparison between the experimental stages
Comparative analysis of the F0 parameter between the four stages of the experiment showed a general tendency for this parameter to increase in all the plants analysed after treatment with short-term heat stress and under field conditions, with the exception of FR, where a decrease in F0 was observed at the IV stage, indicating better stress tolerance. In FC plants, the increase in F0 value was observed between III and IV stage, indicating impairment of the photosynthetic apparatus under field conditions. Moreover, in FC, a decrease in Fv and Fm was observed 1 week after the heat stress treatment, indicating a better stress tolerance of the in vitro regenerated plants. Although a decrease in Fv/Fm was observed in all the plants studied at the II (heat stress) and IV (field conditions) stages, the greatest statistically significant differences were observed in male and female control plants obtained from seeds compared to in vitro regenerated plants, demonstrating the better stress tolerance of the latter. The reason for this result could be due to changes at the cellular level, namely the susceptibility of cells to somaclonal variation.Testing plants for somaclonal variation, has been used to obtain disease-resistant crop species, e.g. alfalfa, maize, sugarcane and tomato. Somaclonal variability results from pre-existing mutations in explant cells, but much of the variability is induced during the cell culture cycle and is responsible for chromosomal changes (Hammerschlag 1990). However, we cannot conclude this based on these studies, as more in-depth studies at the molecular and cytological level are still required.
It has been shown that under heat stress conditions the total chlorophyll content was reduced. This could be at least partly due to the inhibition of enzymes involved in chlorophyll biosynthesis. In a study on Rumex acetosa, ROS was found to influence the perturbations of the Chl a to b ratio, because under stress conditions Chl a is the main substrate of Chl b biosynthesis (Biczak and Pawłowska 2016). Similar results were obtained in R. thyrsiflorus during our experiment, where the decrease in the ratio of Chl a/b was observed after a short-term treatment with high temperatures. Interestingly, at the IV stage of the experiment (field conditions), Chl a/b was highest in the leaves of MR and FR compared to the plants germinated from seeds, proving that they are better adapted to field conditions. In the present study, in the III stage of the experiment, i.e. recovery (7 days after heat stress) the content of total Chl decreased compared to stage I and II. Similar results were obtained in Sorghum under heat stress (Gosavi et al. 2014). Under field conditions, carotenoids appeared to be the dominant pigment group due to the higher intensity of sunlight. Carotenoids dissipate excess photon energy, protecting chlorophylls from photooxidation (Jo et al. 2008). It is interesting that the content of carotenoids in R. thyrsiflorus MR and FR represents a kind of “pre-adaptation” to field conditions with strong sunlight.
SOD and CAT activities
There is limited data on the relationship between sex and antioxidant enzyme activity. In the present study: MnSOD, FeSOD, Cu/ZnSOD I and Cu/ZnSOD II were identified for the first time in Rumex thyrsiflorus. The presence of Cu/ZnSOD at each stage, could be due to the fact that it is the only isoform of SOD found in multiple locations in the plant cell: in the cytosol, chloroplasts, peroxisomes, apoplast, and also in the cell nucleus (Sheng et al. 2014; Ogawa et al. 1996). The presence of the FeSOD form was only detected in female and male plants obtained from seeds (control plants) at the II stage of the experiment and enabled them to cope better with direct exposure to a stress factor-at this stage plants regenerated in vitro showed a reduction in PSII activity.
CAT activity was observed in leaves of plants regenerated in vitro and control plants of both sexes at all stages of the experiment, excluding MR and FR at stage IV (field conditions). The absence of CAT activity in leaves of female and male plants regenerated in vitro under field conditions might indicate that the detection method used is not accurate enough or that the function of CAT is replaced by other H2O2-degrading enzymes, for example peroxidases. Such a phenomenon was observed in Jatropha curcas where catalase activity decreased with increasing water deficit (Sofo et al. 2015). Chen et al. (2015) observed that the increase of ROS as signalling molecules affected the expression of antioxidant systems and the activity of antioxidant enzymes: SOD and ascorbate peroxidase (APX) in females. Robakowski et al. (2018) concluded that larger Taxus baccata female reproductive effort compared with males was not at the cost of photochemical capacity but may to some extent be due to differences between the sexes in the ability to protect the photosynthetic apparatus with antioxidants against photoinhibition. Tolerance to high temperatures varies between species. In the wheat study, increased activity of all antioxidants tested was found at 35°C, but the activity of POX continued to increase with increasing temperature. In contrast, the activity of CAT gradually decreased, suggesting that plants have a species-specific tolerance threshold (Sarkar 2016).
Conclusion
The complex analysis of selected physiological and biochemical aspects of sex-related heat stress response was carried out for the first time in the dioecious model species Rumex thyrsiflorus. The results showed that high-temperature stress response depended on the sex and the origin of the plants, i.e. in vitro regenerated plants versus plants obtained from seeds. Even short-term heat stress led to differences in photosynthetic efficiency.
In general, in vitro regenerated plants showed a reduction in PSII activity when directly exposed to a stress factor. However, in contrast to plants from seeds, they efficiently adapted to recovery conditions within 1 week after termination of the stress factor. The regenerants also acclimatised well to field conditions and in this case showed better efficiency of the photosynthetic apparatus and greater stress tolerance compared to seed-derived control plants. This was also reflected in the Chl a/b ratio, which was highest at this stage in the leaves of the male and female regenerants. Analysis of the main photosynthetic parameters showed that male plants derived from seeds were less sensitive to heat stress and showed greater stability of the photosynthetic apparatus in response to high temperature stress than female plants derived from seeds.
In the present study the forms of SOD: manganese (MnSOD), iron (FeSOD), and two copper-zinc isoforms (Cu/ZnSOD I and Cu/ZnSOD II) were identified for the first time in Rumex thyrsiflorus.
Sex-specific differences in adaptability to environmental stress are still poorly understood, although studying the stress adaptation mechanisms of male and female dioecious plants is essential to understand the evolution of these species in the context of global warming. In our opinion, the results of these studies can be applied in agriculture and are also a good starting point for phytochemical studies on the relationship between sex-specific stress response and the production of secondary metabolites, which are currently being initiated.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ahuja I, de Vos RC, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15(12):664–674. https://doi.org/10.1016/j.tplants.2010.08.002
Baker NR, Rosenquist E (2004) Application of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621. https://doi.org/10.1093/jxb/erh196
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Biczak R, Pawłowska B (2016) Effect of the quaternary ammonium salts, tetraethylammonium halide on Rumex acetosa L., Chenopodium album L. and Galinsoga parviflora Cav.: inhibition of growth and changes in assimilation pigments content in plants. Folia Pomer Univ Technol Stetin Agric Aliment Pisc Zootech 328(39)3:35–52.
Błocka-Wandas M, Sliwinska E, Grabowska-Joachimiak A, Musial K, Joachimiak AJ (2007) Male gametophyte development and two different DNA classes of pollen grains in Rumex acetosa L., a plant with an XX/XY1Y2 sex chromosome system and a female–biased sex ratio. Sex Plant Reprod 20:171–180. https://doi.org/10.1007/s00497-007-0053-9
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1/2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Camejo D, Rodríguez P, Morales A, Dell’Amico JM, Torrecillas A, Alarcón JJ (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162(3):281–289. https://doi.org/10.1016/j.jplph.2004.07.014
Chen J, Duan BL, Wang ML, Korpelainen H, Li CY (2014) Intra- and inter-sexual competition of Populus cathayana under different watering regimes. Funct Ecol 28:124–136. https://doi.org/10.1111/1365-2435.12180
Chen L, Hu X, Yang W, Xu Z, Zhang D, Gao S (2015) The effects of arbuscular mycorrhizal fungi on sex-specific responses to Pb pollution in Populus cathayana. Ecotoxicol Environ Saf 113:460–468. https://doi.org/10.1016/j.ecoenv.2014.12.033
Field DL, Pickup M, Barrett SCH (2013) Ecological context and metapopulation dynamics affect sex–ratio variation among dioecious plant populations. Ann Bot 111:917–923. https://doi.org/10.1093/aob/mct040
Gawel NJ, Jarret RL (1991) A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol Biol Rep 9(3):262–266. https://doi.org/10.1007/BF02672076
Gosavi GU, Jadhav AS, Kale AA, Gadakh SR, Pawar BD, Chimote VP (2014) Effect of heat stress on proline, chlorophyll content, heat shock proteins and antioxidant enzyme activity in sorghum (Sorghum bicolor) at seedlings stage. Indian J Biotechnol 13:356–363
Grabowska-Joachimiak A, Kwolek D, Kula A, Marciniuk P (2012) Fluorescent banding pattern and species-specific DNA marker in Rumex thyrsiflorus Fingerh. Cytogenet Genome Res 137(1):70–77. https://doi.org/10.1159/000339383
Hammerschlag FA (1990) Resistance responses of plants regenerated from peach callus cultures to Xanthomonas campestris pv. pruni. J Amer Soc Hort Sci 115:1034–1037. https://doi.org/10.21273/JASHS.115.6.1034
Hao L, Chena L, Zhua P, Zhanga J, Zhanga D, Xiaoa J, Xua Z, Zhanga L, Liua Y, Lia H, Yanga H, Caoc G (2020) Sex-specific responses of Populus deltoides to interaction of cadmium and salinity in root systems. Ecotoxicol Environ Saf 195:110437. https://doi.org/10.1016/j.ecoenv.2020.110437
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96(15):8762–8767. https://doi.org/10.1073/pnas.96.15.8762p
Jo E-A, Tewari RK, Hahn E-J, Paek K-Y (2008) Effect of photoperiod and light intensity on in vitro propagation of Alocasia amazonica. Plant Biotechnol Rep 2:207–212. https://doi.org/10.1007/s11816-008-0063-6
Juvany M, Munné-Bosch S (2015) Sex-related differences in stress tolerance in dioecious plants: a critical appraisal in a physiological context. J Exp Bot 66(20):6083–6092. https://doi.org/10.1093/jxb/erv343
Kalaji HM, Bosa K (2011) Handy pea, pocket pea & pea plus software. Operation Manual. Setup installation & maintenance. Hansatech Instruments ltd., King’s Lynn
Korpelainen H (2002) A genetic method to resolve gender complements investigations on sex ratios in Rumex acetosa. Mol Ecol 11(10):2151–2156. https://doi.org/10.1046/j.1365-294X.2002.01593.x
Kwolek D, Joachimiak AJ (2011) Seed sexing revealed female bias in two Rumex species. Acta Soc Bot Pol 80(2):93–97. https://doi.org/10.5586/asbp.2011.028
Lichtenthaler HK (1987) Chlorophylls and carotenoids, the pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterisation by UV–VIS. Curr Prot Food Analyt Chem 1(1):F431–F438. https://doi.org/10.1002/0471142913.faf0403s01w
Liu M, Korpelainen H, Li C (2021) Sexual differences and sex ratios of dioecious plants under stressful environments. J Plant Ecol 14(5):920–933. https://doi.org/10.1093/jpe/rtab038
Mariotti B, Manzano S, Kejnovsky E, Vyskot B, Jamilena M (2009) Accumulation of Y-specific satellite DNAs during the evolution Rumex acetosa sex chromosomes. Mol Genet Genomics 281(3):149–259. https://doi.org/10.1007/s00438-008-0405-7
Miszalski Z, Ślesak I, Niewiadomska E, Bączek-Kwinta R, Lüttge U, Ratajczak R (1998) Subcellular localization and stress responses of superoxide dismutase isoforms from leaves in the C3-CAM intermediate halophyte Mesembryanthemum crystallinum L. Plant Cell Environ 21(2):169–179. https://doi.org/10.1046/j.1365-3040.1998.00266.x
Munné-Bosch S (2015) Sex ratios in dioecious plants in the framework of global change. Environ Exp Bot 109:99–102. https://doi.org/10.1016/j.envexpbot.2014.08.007
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:437–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Navajas-Pérez R, De La Herrán R, Jamilena M, Lozano R, Rejón CR, Rejón MR, Garrido-Ramos MA (2005) Reduced rates of sequence evolution of Y-linked satellite DNA in Rumex (Polygonaceae). J Mol Evol 60:391–399. https://doi.org/10.1007/s00239-004-0199-0
Navajas-Pérez R, Schwarzacher T, De La Herran R, Ruiz Rejón C, Ruiz Rejón M, Garrido-Ramos MA (2006) The origin and evolution of the variability in a Y-specific satellite-DNA of Rumex acetosa and its relatives. Gene 368:61–71
Ogawa K, Kanematsu S, Asada K (1996) Intra-and extra-cellular localization of cytosolic CuZn-superoxide dismutase in spinach leaf and hypocotyls. Plant Cell Physiol 37:790–799. https://doi.org/10.1093/oxfordjournals.pcp.a029014
Orbán-Gyapai O, Forgo P, Hohmann J, Vasas A (2017) Phytochemical investigation of Rumex thyrsiflorus Fingerh. Acta Biol Hung 68(2):232–236. https://doi.org/10.1556/018.68.2017.2.10
Pérez-Jiménez M, Pérez-Tornero O (2020) In vitro plant evaluation trial: reliability test of salinity assays in citrus plants. Plants 9:1352. https://doi.org/10.3390/plants9101352
Pospíšilová J, Tichá I, Kadleček P, Haisel D, Plzáková Š (1999) Acclimatization of micropropagated plants to ex-vitro conditions. Biol Plant 42:481–497
Renner SS (2014) The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am J Bot 101(10):1588–1596. https://doi.org/10.3732/ajb.1400196
Robakowski P, Pers-Kamczyc E, Ratajczak E, Thomas PA, Ye Z-P, Rabska M, Iszkuło G (2018) Photochemistry and antioxidative capacity of female and male Taxus baccata L. acclimated to different nutritional environments. Front Plant Sci 9:742. https://doi.org/10.3389/fpls.2018.00742
Rychlewski J, Zarzycki K (1975) Sex ratio in seeds of Rumex acetosa L. as a result of sparse or abundant pollination. Acta Biol Crac Ser Bot 18:101–113
Sarkar J, Chakraborty B, Chakraborty U (2016) Temperature stress induced antioxidative and biochemical changes in wheat (Triticum aestivum L.) cultivars. J Plant Stress Physiol 2:22–30. https://doi.org/10.19071/jpsp.2016.v2.3076
Sharma DK, Anderson SB, Ottosen C-O, Rosenqvist E (2015) Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol Plant 153(2):284–298. https://doi.org/10.1111/ppl.12245
Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller A-F, Teixeira M, Valentine JS (2014) Superoxide dismutases and superoxide reductases. Chem Rev 114:3854–3918. https://doi.org/10.1021/cr4005296
Ślesak I, Hałdaś W, Ślesak H (2006) Influence of exogenous carbohydrates on superoxide dismutase activity in Trifolium repens L. explants cultured in vitro. Acta Biol Cracov Bot 48(1):93–98
Ślesak H, Góralski G, Kwolek D, Dziedzic K, Grabowska-Joachimiak A (2015) Male adventitious roots of Rumex thyrsiflorus Fingerh. as a source of genetically stable micropropagated plantlets. Plant Cell Tiss Organ Cult 123(1):193–203. https://doi.org/10.1007/s11240-015-0826-z
Ślesak H, Dziedzic K, Kwolek D, Cygan M, Mizia P, Olejniczak P, Joachimiak AJ (2017) Female versus male: Rumex thyrsiflorus Fingerh. under in vitro conditions. Does sex influence in vitro morphogenesis? Plant Cell Tiss Organ Cult 129:521–532. https://doi.org/10.1007/s11240-017-1197-4
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16(6):13561–13578. https://doi.org/10.3390/ijms160613561
Szymańska R, Ślesak I, Orzechowska A, Kruk J (2017) Physiological and biochemical responses to high light and temperature stress in plants. Environ Exp Bot 139:165–177. https://doi.org/10.1016/j.envexpbot.2017.05.002
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44(1):301–305. https://doi.org/10.1016/0003-2697(71)90375-7
Zhai F-f, Li H-d, Zhang S-w, Li Z-j, Liu J-x, Qian Y-q, Ju G-s, Zhang Y-x, Liu L, Han L, Sun Z-y (2020) Male and female plants of Salix viminalis perform similarly to flooding in morphology, anatomy, and physiology. Forests 11(13):321. https://doi.org/10.3390/f11030321
Zhang S, Han S, Yang W, Wei H, Zhang M, Qi L (2010) Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of Larix leptolepis. Plant Cell Tiss Organ Cult 100(1):21–29. https://doi.org/10.1007/s11240-009-9612-0
Zhang Y, Virjamo V, Sobuj N, Du W, Yin Y, Nybakken L, Guo H, Julkunen-Tiitto R (2018) Sex-related responses of European aspen (Populus tremula L.) to combined stress: TiO2 nanoparticles, elevated temperature and CO2 concentration. J Hazard Mater 352:130–138. https://doi.org/10.1016/j.jhazmat.2018.03.031
Żuk J (1963) An investigation on polyploidy and sex-determination within the genus Rumex. Acta Soc Bot Pol 32(1):5–72. https://doi.org/10.5586/asbp.1963.001
Acknowledgements
We would like to thank dr. Agnieszka Marek from the Institute of Botany at the Jagiellonian University in Kraków for technical assistance in SOD and CAT analyses. The article is dedicated to the celebration of “2022-Year of Botany” in Poland.
Funding
The work was partially supported by the statutory research funds N18/DBS/000002 (2021) of the Institute of Botany, Faculty of Biology, Jagiellonian University in Krakow.
Author information
Authors and Affiliations
Contributions
The following declarations about authors contributions to the research have been made: in vitro culture experiments, molecular, physiological and biochemical analyses, data interpretation, draft preparation: KG; data interpretation, proofreading the manuscript: IŚ; design of the study, physiological analysis, data interpretation, statistical analysis, writing a manuscript: HŚ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Patricia Marconi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gozdur, K., Ślesak, I. & Ślesak, H. Photosynthetic and antioxidant activity in the sex-related heat stress response of the dioecious species Rumex thyrsiflorus Fingerh.. Plant Cell Tiss Organ Cult 152, 151–165 (2023). https://doi.org/10.1007/s11240-022-02397-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02397-z