Abstract
Pierce’s disease is a deadly disease of grapevines caused by the bacterial pathogen Xylella fastidiosa (Xf). A Pierce’s disease resistance locus from Vitis arizonica/candicans b43-17 segregated as a single dominant gene and mapped as PdR1a and PdR1b in two F1 sibling selections. The physical mapping of the PdR1b allele allowed the identification of five ORFs of the Leucine-Rich Repeat Receptor Kinase gene family. Two ORFs: V.ari-RGA14 and V.ari-RGA18 were used to transform embryogenic callus of V. vinifera Chardonnay (CH) and Thompson Seedless (TS) and V. rupestris St George (SG) via Agrobacterium tumefaciens. Regenerated plants were inoculated with Xf under greenhouse conditions. Genetic transformation with RGA14 and 18 did not generate resistance in CH and TS, although some lines of CH showed significantly lower stem bacterial concentration and/or exhibited reduced symptoms. In transgenic SG14, improved regrowth was accompanied with lower bacterial titers and decreased pectin lyase and ß-1,3-glucanase 3 gene expression. The limited effects of the transgenes on PD resistance could be explained by the lack of suitable partners or the presence of susceptibility factors that could not be overcome under these experimental conditions. The involvement of RGA17 in b43-17 resistance to Xf should not be discarded.
Key message
The expression of two candidate genes for Pierce’s disease resistance in Vitis arizonica/candicans b43-17 had limited effect in the resistance of susceptible vinifera genotypes to the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pierce’s disease (PD) of grapevines, caused by the bacterial pathogen Xylella fastidiosa (Xf), is a severe problem for grape cultivation throughout the southern areas of North America. The European grape Vitis vinifera, from which most of the wine, table and raisin grape cultivars are derived, is highly susceptible to PD, and succumb to the disease within 5 years after infection. Pierce’s disease symptoms include leaf scorching, petioles that remain attached after leaf fall and irregular cane lignification that produce patches of green, surrounded by mature, brown tissue (Varela et al. 2001). This pathogen is vector-transmitted from one plant to another by various xylem sap-feeding insects (Chatterjee et al. 2008). Following infection, the bacteria form biofilm-like colonies that together with host produced tyloses and polysaccharides plug water movement in host xylem tissue (Chatterjee et al. 2008; Sun et al. 2013). Several studies have revealed key aspects of Xf pathogenicity. Hxf proteins (hemagglutinin adhesins) have been found to be involved in Xf virulence and cell-to-cell aggregation (Guilhabert and Kirkpatrick 2005). The rpfF gene was reported to encode the synthase for a diffusible signal factor (DSF) that upregulates factors required for biofilm formation and reduces lateral and longitudinal movement in the plant (Newman et al. 2004; Lindow et al. 2014). Other studies have shown that the Xf polygalacturonase (PG) and several ß-1,4-endoglucanases (EGases) may contribute to the degradation of the pit membranes that limit intervessel migration (Roper et al. 2007). A long chain O-antigen was found to delay the elicitation of innate immunity by shielding lipopolysaccharides that trigger early plant recognition (Rapicavoli et al. 2018). Nascimento et al. (2016), added lipase/esterase LesA to the list of virulence factors and suggested that LesA secretion and accumulation in the margins of infected grapevine leaves leads to leaf chlorosis and marginal necrosis. In addition, host structural polysaccharides produce dramatic changes in Xf gene expression required for vector transmission (Killiny and Almeida 2009).
Plant response mechanisms were explored by Choi et al. (2013), who studied the interaction of drought stress and disease in Cabernet Sauvignon. They found that the transcriptional host response activated by Xf infection differs from that of healthy plants to drought stress. They detected that the expression of genes known to function in water-stress tolerance and several pathogen-induced genes was strongly activated by the interaction of Xf infection and drought. Zaini et al. (2018) characterized PD infection in Thompson Seedless. They concluded that infected Thompson Seedless: (1) activated both pathogen- and damage-associated molecular pattern (PAMP/DAMP)-triggered immunity (PTI), but displayed reduced downstream salicylic acid-mediated immune response; (2) was unable to generate antioxidant strategies capable of preventing oxidative stress during disease development; and (3) strongly upregulated various cell wall remodeling and lignification enzymes, decreasing sap flow and thereby water and nutrient availability.
Resistance to PD exists in American Vitis species and has been introgressed into many hybrid cultivars (Krivanek et al. 2005a). Mortensen (1968) evaluated the inheritance of PD resistance from V. aestivalis ssp. smalliana, V. simpsonii and V. shuttleworthii in Florida under field conditions in high disease pressure environments. Plant vigor and longevity measured over a 5-year period were used to determine whether a genotype was resistant or susceptible. Upon evaluation of qualitative segregation ratios of progeny derived from several controlled crosses, Mortensen concluded that resistance was dominant to susceptibility and suggested that complementary gene action among three independent genes could best explain the results. Clarke et al. (2003) also reported resistance in Muscadinia rotundifolia ‘Fry’ and ‘Carlos’. Riaz et al. (2018, 2020), carried out an extensive analysis of PD resistance in Vitis species and added Vitis candicans and Mexican V. cinerea accessions to the list. Among southwestern grape species, Vitis arizonica accessions displayed strong PD resistance despite genetic variation and V. girdiana and V. treleasei showed moderate resistance. Although the mechanisms by which these native grape species resist PD is unknown, it is plausible that native species must have acquired resistance through co-evolution with Xf over millennia. Deeper knowledge of resistance in Vitis arizonica accessions has accumulated over the years. One of them, Vitis arizonica/candicans b43-17 is the source of PD resistance in 5 recently released grape varieties from the University of California (Walker et al. 2021). b43-17 is homozygous resistant to Xf (Krivanek et al. 2005a, Krivanek et al. 2006, Riaz et al. 2008) and was introgressed into V. vinifera using marker assisted selection and a greenhouse-based assay to evaluate disease development (Riaz et al. 2009). Tylose development in PD-resistant grapevines carrying one of the 2 alleles above mentioned was investigated by Sun et al. (2013). Tyloses are outgrowths into a vessel lumen from living parenchyma cells that are adjacent to the vessel. Quantitative comparison of vascular occlusions in infected vines showed that over 60% of the vessels of the susceptible cultivars were blocked, while only 5% to 27% were blocked in inoculated shoots of resistant genotypes. In contrast to the systemic spread in PD-susceptible genotypes, bacteria were restricted to internodes near the inoculation points in the PD-resistant grapevines.
The resistance locus in b43-17, was mapped to chromosome 14 as PdR1a in the F1 selection F8909-17 and as PdR1b in full sib F1 selection F8909-08 (Riaz et al. 2006, 2008). The genetic maps developed from these studies were further used to produce physical maps. The assembled BAC clones that represent the PdR1a and b haplotypes showed that there is complete sequence homology between haplotype a and b of the PdR1 locus. The genomic location of the PdR1c locus from V. arizonica b40-14 is also similar to the PdR1a and PdR1b loci (Walker and Riaz 2013, 2014; Walker et al. 2015), however the comparison of the PdR1 region from b43-17 and b40-14 showed sequence divergence for the region of resistant genes and for a number of transposable elements, indicating significant differences between the two accessions (Riaz et al. 2019).
The genetic window for the PdR1b locus has been narrowed to 82 Kb between markers SSR-1b4-1 and SSR-Orf18-19–1. Five ORFs within this region, associated with disease resistance, were designated resistance gene analog (RGA) 14, 15, 16, 17 and 18 (Walker et al. 2015). Molecular and functional characterization of these candidates will help to identify and validate the candidate gene involved in conferring resistance to PD and will broaden our knowledge of the mechanisms involved in this plant response. The objectives of this study were: (a) to carry out comparative sequence analysis of the b43-17 candidate genes with reference V. vinifera PN40024; (b) analyze their expression in contrast to susceptible Chardonnay; and (c) evaluate the PD resistance of susceptible genotypes Chardonnay, Thompson Seedless and V. rupestris St. George transformed with these genes under the regulation of their native promoters.
Materials and methods
Plant material
Plants of b43-17, Chardonnay (CH), Thompson Seedless (TS), V. rupestris St George (SG) and transgenics were clonally propagated, potted and grown under greenhouse conditions. Potted plants were randomly distributed on greenhouse benches and inoculated with Xf as previously described (Krivanek et al. 2005b).
Manual annotation
The 82 kb region delimited by the markers that flank the PdR1b resistance locus (SSR-1b4-1 and SSR-Orf18-19–1) was annotated through multiple sequence alignment against PN40024 using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/); search for repetitive elements with the Giri database (https://www.girinst.org/censor/index.php), blast with Phytozome 13 (https://phytozome-next.jgi.doe.gov/info/Vvinifera_v2_1) and analysis of predicted proteins using the Interpro database (https://www.ebi.ac.uk/interpro/).
Genetic transformation and Xf inocultation
Based on manual annotation results, fragments that contain RGA14 or RGA18 plus ∼3 Kb upstream and ∼1 Kb downstream sequences were selected for synthesis and cloning into pCLB2301NK (Feechan et al. 2013) at Genewiz Inc. These plasmids also contained a 35S:mGFP5-ER reporter cassette and a kanamycin-selectable marker gene. Agrobacterium tumefaciens strain EHA105 pC32 was chemically transformed with pCLB2301NK-14 or pCLB2301NK-18 (Fig. 1) and subsequently used to transform embryogenic calli of CH, TS and SG as previously described (Agüero et al. 2006). Transformation was verified through PCR with primers that bind the promoter and coding regions of RGA14 or RGA18 (Table S1) and by fluorescence microscopy. Transgenic and untransformed control plants were acclimated to greenhouse conditions for testing. Five to six replicates of each line were produced from green cuttings and trained as single shoots. These plants were needle inoculated (Hopkins 1980; Krivanek et al. 2005b) with the Beringer strain of Xf. Stems were inoculated twice about 10–15 cm above the base with a total of 20 uL of cell suspension standardized with ddH2O to an absorbance at 600 nm of 0.25 (approximately 6 × 108 CFU/ml as determined by culture plating).
Gene expression
Plants grown under the same greenhouse conditions used for Xf inoculation (Krivanek et al. 2005b) were sampled 3 weeks post-inoculation (wpi) by taking 0.5 g sections of stem tissue from 30 cm above the point of inoculation (poi). Total RNA was extracted using a CTAB method (Blanco-Ulate et al. 2013). RNA pellet was further purified using the Quick-RNA Miniprep Kit (Zymo Research). cDNA was prepared using M-MLV RT (Promega). Gene specific primers were designed for each candidate gene (Tables S2 and S3). qRT-PCR was performed on a StepOnePlus PCR System using Fast SYBR Green Master Mix (Applied Biosystems). All qRT-PCR reactions were performed as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. The 2-∆∆CT method (Livak and Schmittgen 2001), using the grape actin gene as reference (Jones et al. 2014), was applied to analyze expression levels. Fragments were sent to Quintara Biosciences for cleanup and sequencing with the same primers that amplified the fragment.
Transcripts encoding pathogenesis related proteins and anti-microbial compounds related to Xf infection (Zaini et al. 2018) were also analyzed.
Disease evaluations by greenhouse-based assay
Plants were sampled 12 wpi by taking 0.5 g sections of stem tissue from 30 cm above the poi. Samples were ground and tested by ELISA following published procedures (Krivanek and Walker 2005). Leaf scorching, cane maturation and shoot regrowth were also recorded (Krivanek et al. 2005b).
Statistical analysis
Data were analyzed with analysis of variance (ANOVA) followed by DGC α = 0.05. To achieve normal distribution, the data points of cells per milliliter concentration and gene relative expression were natural log transformed. Statistical analyses were performed using the InfoStat software program, version 2.0 (Di Rienzo et al. 2014).
Results
Annotation of candidate genes
Molecular markers delimited the PdR1b resistance locus within a genetic region of 82 Kb in BAC clone H69J14. Analysis of this region resulted in the identification of 5 ORFs showing characteristics associated with disease resistance. They were designated V.ari-RGA14-15–16-17 and 18 (Table 1). Three additional ORFs encoding truncated versions of a TMV resistance protein N and two partial retrovirus-related proteins were also found in the region but disregarded as potential candidates.
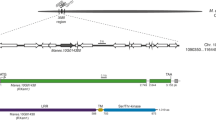
Sequence analysis showed that RGA14 is very similar to RGA18, except that RGA18 lacks the first 252 bp of sequence that is part of RGA14. However, RGA14 has 4 ATG codons, in the same reading frame, closely located in the 5' end and a functional protein could be shorter and quasi- identical to the one coded by RGA18. A TATA box is present 25 bp upstream from the third start codon (RGA14c). Interestingly, RGA14 is interrupted by retrotransposon Gret1 in PN40024. RGA15 is indistinguishable to its homologous in PN40024 and b40-14, while 16 is much shorter. Homologs of RGA15 and 16 in PN, g00040 and g00050, have only one transcript (splice variant) and no introns. RGA17 and 18 have high homology with genes in a duplicated region of PN40024 that contains 2 genes with no introns (g00060 and g00070) in the first copy and a larger gene with 2 exons (g00080) in the second copy. In the latter, RGA18 overlaps with exon 1 and RGA17 overlaps with exon 2 of g00080. A VvinBV_compBsc2 integrated virus element is found 768 bases upstream the start codon of RGA18 (Fig. 2). ORF sequences found outside the 82 Kb window were also highly similar. Protein predictions revealed that all five candidates exhibit leucine-rich repeats (LRRs) and feature transmembrane domains, except for RGA17. RGA16 and the three longest possible translations of RGA14 lack a signal peptide in the amino terminal of the protein (Fig. 2).
a The b43-17 region containing RGA14 to 18 and the homologous region of Vitis vinifera PN40024 v2.1. RGA1-13 and 19–21, in the same family, are outside this region. Genes are represented by blue arrows. Orange hexagons represent repetitive elements Gret1 and VvinBV_compBsc2; b Phylogenetic relationship of b43-17 RGA14-18 deduced proteins. The phylogenetic tree was constructed by MEGA 11.0 using the Maximum Likelihood Method (1000 bootstrap); c Alignment of the deduced amino acid sequences of the five RGA sequences using Muscle (MEGA 11.0) with the default options. The gray shade shows identical residues. The yellow, black, diagonal stripes and dotted horizontal bars under the sequences indicate the extracellular domain, signal peptide, transmembrane domain and intracellular domain respectively. Domains and motifs were predicted using Interproscan and SignalP5.0. The four N terminal methionines in RGA14 are underlined. (Color figure online)
Results from annotation made RGA14 and RGA18 the targets for transgenic experiments. We hypothesized that RGA14 was responsible for resistance observed in b43-17 because its homologous is disrupted in PN40024. Alternatively, regulation of the expression of RGA18 could have been modified with the insertion of VvinBV_compBsc2 integrated virus element in its promoter region.
Expression of candidate genes in resistant b43-17 and susceptible CH
In parallel with Xf inoculations of transgenic plants, gene expression was analyzed in 3 biological replicates of b43-17 and CH controls. PCR amplifications of cDNA synthesized from infected shoots produced the expected bands, that were cleaned and sequenced (Fig. 3). Primers designed to amplify both RGA14 and 18, resulted in the amplification of fragments that comprise sequences identical to RGA14 (within the genetic window) and RGA12 (outside the genetic window). In CH PD, the same primers amplified fragments 96% homologous to g00080. RGA15 primers produced the predicted size bands in b43-17 PD and CH PD, with identical sequences to RGA15 and 99.4% homology with g00040, respectively. Primers designed for RGA16 only amplified in CH PD, the PCR product showing 98.5% homology with g00050, while primers for RGA17 only produced the expected sequence in b43-17 PD (Fig. 3). These results imply that in infected plants, susceptible CH would differ from resistant b43-17 in the absence of RGA14 and RGA17 transcripts. The homologous to RGA18 might be expressed in CH but not in b43-17.
PCR amplifications using infected Chardonnay (CH) and b43-17 (b) cDNA as template. 1 kb ladder (New England Biolabs) is in lane 1, with the 3 kb fragment having increased intensity to serve as a reference band. The expected size of the bands produced by each pair of primers appears at the bottom of the gel. Bands of the expected size were also obtained with genomic DNA except in CH14 and 17 (data not shown). 14/18 indicates a primer combination that hybridizes with sequences in RGA14 and RGA18. RT-PCR products were cleaned and sequenced
qPCR experiments showed a slight upregulation of RGA15 in b43-17 PD and its homologous gene in CH PD (Fig. 4). A more significant increase of RGA17 and RGA14 was also found in infected b43-17. Sequencing of qPCR products showed that RGA14 primers amplified a non-specific fragment in CH. Similarly, RGA18 primers amplified a non-specific product in b43-17. Primers could have cross reacted with transcripts from similar LRR genes located outside the 82 Kb window, producing unspecific amplicons.
Relative expression of RGA14, 15, 17, 18 measured by qPCR, in healthy (H) and infected (PD) Chardonnay (CH) and b43-17. Fold changes were measured relative to the levels detected in healthy b43- 17. P1Q1 primers amplify the 5’ region of RGA14. RGA16 was not analyzed. Results are reported as means of three biological replicates and three technical replicates. Bars represent standard errors. qPCR products were cleaned and sequenced
Nevertheless, overall expression levels in CH were higher than b43-17, but response to Xf infection was more drastic in b43-17. qPCR amplification with primers P1Q1, which hybridizes within the unique 5’ region of RGA14, was almost undetectable (Fig. 4).
Transgenic plants transformed with RGA14 and RGA18
After transformation was verified through PCR and GFP expression by fluorescence (Figures S1 and S2), 43 transgenic lines of CH and TS (10–11 lines per genotype × 2 constructs) were evaluated for disease development. All transgenic lines displayed PD symptoms, although with different degrees of intensity (Fig. 5). Some lines exhibited reduced symptoms or lower bacteria concentrations, but none reached the levels of the resistant biocontrols with either transgene (Tables 2 and 3).
Plant regeneration from transgenic SG was more challenging and fewer independent lines could be tested against Xf. A marginally better outcome was observed in SG transformed with pCLB2301NK-14 (Table 4). They displayed lower cane maturity index (CMI) and leaf scorching and, more interestingly, active shoot regrowth after cut back for sampling 12 weeks after inoculation, which could signal a superior hydraulic conductivity (Fig. 6). Of four SG transformed with RGA18: two were dwarf and could not be propagated and the other two exhibited bacterial concentrations higher than the control.
To connect the genetic basis of resistance with pathogen related responses, we also analyzed transcript levels of pathogen related proteins linked to Xf infection. Among 15 genes known to be upregulated in infected TS (Zaini et al. 2018), PR-1, ß-1,3-glucanase 3 and pectin lyase were significantly different in transgenic SG 14–2, the line showing more tolerance (Fig. 7). The analysis of these three genes in b43-17 and the other transgenic lines showed that they were also expressed at lower levels relative to SG untransformed, except for glucanase in line SG 14–6. Pectin-lyase was highly expressed in line SG 14–5 which harbored a bacterial population similar to the untransformed control (Fig. 8).
Relative expression of fifteen plant defense genes measured by qPCR, in infected untransformed (SG UN) and transgenic SG 14–2. Genes are (1) Pathogenesis related protein 1 (PR-1); (2) Thaumatin (PR5); (3) EPC3-3 chitinase (PR-8); (4) Heat shock protein HSP18; (5) Heat shock protein HSP17; (6) Heat shock factor HSF4; (7) Nucleoredoxin 1 (NRX1); (8) Peroxidase; (9) Ferritin-5; (10) ß-1,3-glucanase 3; (11) Sucrose synthase (SuSy); (12) Xyloglucan hydrolase XTH 32; (13) Expansin-like B1; (14) Pectin lyase; (15) UDP-Glycosyltransferase (UGT). Fold changes were measured relative to the levels detected in Nucleoredoxin 1. Results are reported as mean of three biological replicates and three technical replicates. Bars represent standard errors
Relative expression of a PR-1, b ß-1,3-glucanase 3 and c pectin lyase measured by qPCR, in healthy (H) and infected (PD) b43-17, untransformed (SG UN) and transgenic SG lines 14–2, 14–4, 14–5 and 14–6. Fold changes were measured relative to the levels detected in healthy b43-17. Results are reported as means of three biological replicates and three technical replicates. Bars represent standard errors. Different letters mean statistically significant differences at p < 0.05
Discussion
Annotation and expression of candidate genes
Receptor-like kinases (RLKs) and receptor-like proteins (RLPs) are membrane-localized proteins with a critical role in PTI through the detection of ligands derived from microbes or cell damage as signs of infection. Ligand perception usually induces receptor homo- or heterodimerization, which initiates cis- and/or trans-phosphorylation of intracellular kinase domains that in turn activate downstream defense pathways (Bentham et al. 2020). Accordingly, pathogens have developed strategies to suppress PTI through the production of effectors that disrupt the PTI signaling. These effectors are sometimes recognized by host intracellular resistance (R) proteins that trigger a second level of defense called effector-triggered immunity (ETI) (Jamieson et al. 2018).
Receptor-like genes encoding proteins with an extracellular LRR domain constitute the most overrepresented family in plant genomes. The LRR-RKs are composed of a transmembrane domain, the LRR extracellular domain, and a cytoplasmic region with the kinase domain. LRR-RLPs are similar to LRR-RKs in structure but lack the cytoplasmic kinase domain (Chakraborty et al. 2019). The presence of LRRs and transmembrane domain and the absence of a kinase domain in the PdR1b candidate genes would put them in the LRR-RLP group. The lack of a kinase domain implies that this type of receptor requires a partner co-receptor to signal (Bentham et al. 2020).
Comparison with susceptible PN40024 sequences led us to choose RGA14 and RGA18 as main candidates for transformations since RGA15 is also present in PN40024, while RGA16 and 17 appear as shorter versions of their PN40024 homologs. RGA14 is very similar to RGA18, however the sequencing of RT-PCR and qPCR products from b43-17 suggested that transcripts are not produced in the latter. On the other hand, the presence of a signal peptide that would target the protein to the plasma membrane (Ben Khaled et al. 2015) was only predicted in the shortest version of RGA14. This could be the main variant, since qPCR with primers at the beginning of the sequence produced inferior expression levels.
Transgenic plants transformed with RGA14 and RGA18
RGA14 and 18 were used for transformation of CH, TS and SG including ∼3 Kb upstream and ∼1 Kb downstream sequences to ensure the control of native regulatory sequences. Genetic transformation with RGA14 and 18 did not generate resistance in transgenic CH and TS, although 2 lines of CH18 showed significantly lower stem bacterial concentration and several lines of CH18 and 14 exhibited reduced symptoms (Tables 2 and 3). TS exhibited the most severe PD expression at 12 weeks post-inoculation and hosted the highest Xf titer of the tested cultivars (Tables 2 and 3). Deyett et al. (2019), also ranked TS as the most susceptible to PD when comparing it to Merlot and Cabernet Sauvignon. Symptoms observed in a preliminary experiment conducted with the first line of transgenic SG14 available for evaluation (SG 14–2) were less severe (data not shown) and plants were kept after ELISA sampling to observe regrowth, which also was improved and correlated with lower bacterial titers (Table 4, Fig. 6). Testing of 5 additional SG14 lines also showed diminished bacterial levels in two of them (SG 14–4 and SG 14–6). Interestingly, previous work in tobacco showed that plants transformed with RGA14 under 35SCaMV promoter had significantly reduced symptoms than the control untransformed plants, although Xf bacterial levels were not reduced (Bistue 2014).
Genetic background plays an important role in gene penetrance. Several R genes remain effective in transgenic heterologous plant species, while others do not retain their proper function, which may be due to lack of specific interaction partners (Gallois et al. 2018). In our case the missing partner could have different degrees of efficiency in V. vinifera and V. rupestris. Interestingly, Feechan et al. (2013) found that the resistance to powdery mildew displayed by transgenic V. vinifera cultivars expressing the MrRUN1 gene was lower than that observed in the genotype from which the MrRUN1 gene was isolated and speculated that this would reflect the minor contributions of other genes. Moreover, the relevance of genetic background has been demonstrated in studies of gene penetrance among the grape varieties carrying the Rpv3-locus that confers resistance to Plasmopara viticola. While some genotypes exhibited high resistance under all conditions, others were less effective in restricting pathogen growth when disease pressure increased. Rvp3 resistance tended to attenuate in late backcross generations and had lower levels in Central Asian table grapes (Foria et al. 2018).
Besides the failure of the plant immune system, there are other host processes that could contribute to the differences observed among genotypes. Hosts can promote susceptibility to pathogen infection by: (i) releasing compounds that attract pathogens and stimulate their attachment and development on the host surface, (ii) contributing to the accommodation of the pathogen inside the plant cell, and (iii) providing nutrients to the pathogen (Lapin and Van den Ackerveken 2013). In Xf, PG and ß-1,4 EGases allow bacteria to degrade the pit membranes that separate adjacent xylem vessels thereby limiting systemic colonization of the host plant. This process releases nutrients for the pathogen (Harakava et al. 2001). Pectin and glucan have also been found to increase cell–cell adhesion and vector transmission and to induce changes in the expression of genes implicated in Xf pathogenicity (Killiny and Almeida 2009). The decreased expression of plant pectin lyase and ß-1,3-glucanase 3 in transgenic SG14 could be the consequence of the presence of the transgene in these lines. Interestingly, Silva et al. 2021 recently established that the pectin-degrading enzyme pectate lyase, is a disease susceptibility factor associated with Botrytis cinerea infections in ripe tomato fruit.
In conclusion, the limited effects of the transgenes on PD resistance could be explained by the lack of suitable partners or the presence of susceptibility factors that can’t be overcome in vinifera/rupestris SG genetic backgrounds under the experimental conditions used in this research. However, the involvement of RGA17 in b43-17 resistance to Xf should not be discarded because its encoded protein, lacking a transmembrane domain, could be secreted and function as a pathogenesis related protein like PGIP (Kalunke et al. 2015; Agüero et al. 2005). On the other side, the lower bacterial load observed in the TS line transformed with both RGA14 and 18 points to the fact that a combination of more than one RLP could be needed. We have included RGA17 in future studies involving knockouts via the CRISPR-Cas9 system in resistant genotype U0505-01 to better understand the role that each of the candidates play in PD resistance.
This research was aimed at understanding the molecular basis of the resistance to PD in b43-17 through the PdR1b allele. In the long term, this knowledge could contribute to a precision genetics approach to develop durable and sustainable resistance through the combination of mechanism-based strategies to improve the plant immune system (Dangl et al. 2013).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Agüero CB, Uratsu SL, Greve C, Powell AL, Labavitch JM, Meredith CP, Dandekar AM (2005) Evaluation of tolerance to Pierce’s disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol Plant Pathol 6:43–51. https://doi.org/10.1111/j.1364-3703.2004.00262.x
Agüero CB, Meredith CP, Dandekar AM (2006) Genetic transformation of Vitis vinifera L. cvs. Thompson Seedless and Chardonnay with the pear PGIP and GFP encoding genes. Vitis 45:1–8
Ben Khaled S, Postma J, Robatzek S (2015) A moving view: subcellular trafficking processes in pattern recognition receptor-triggered plant immunity. Annu Rev Phytopathol 53:379–402. https://doi.org/10.1146/annurev-phyto-080614-120347
Bentham AR, De la Concepcion JC, Mukhi N, Zdrzałek R, Draeger M, Gorenkin D, Hughes RK, Banfield MJ (2020) A molecular roadmap to the plant immune system. J Biol Chem 295:14916–14935. https://doi.org/10.1074/jbc.REV120.010852
Bistue C (2014) Expression of candidate Pierce's disease resistance genes from Vitis arizonica/candicans b43–17 in Nicotiana tabacum cv. sr1. M.S. Thesis University of California, Davis.
Blanco-Ulate B, Vincenti E, Powell AL, Cantu D (2013) Tomato transcriptome and mutant analyses suggest a role for plant stress hormones in the interaction between fruit and Botrytis cinerea. Front Plant Sci 4:142. https://doi.org/10.3389/fpls.2013.00142
Chakraborty S, Nguyen B, Wasti SD, Xu G (2019) Plant leucine-rich repeat receptor kinase (LRR-RK): structure, ligand perception, and activation mechanism. Molecules 24:3081. https://doi.org/10.3390/molecules24173081
Chatterjee S, Almeida RP, Lindow S (2008) Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu Rev Phytopathol 46:243–271. https://doi.org/10.1146/annurev.phyto.45.062806.094342
Choi HK, Iandolino A, da Silva FG, Cook DR (2013) Water deficit modulates the response of Vitis vinifera to the Pierce’s disease pathogen Xylella fastidiosa. Mol Plant Microbe Interact 26:643–657. https://doi.org/10.1094/MPMI-09-12-0217-R
Clarke EE, Ren Z, Lu J (2003) Evaluation of grape germplasm for resistance to Pierce’s disease and glassy-winged sharpshooter. Proc Fla State Hortic Soc 116:32–35
Dangl JL, Horvath DM, Staskawicz BJ (2013) Pivoting the plant immune system from dissection to deployment. Science 341:746–751. https://doi.org/10.1126/science.1236011
Deyett E, Pouzoulet J, Yang J-I, Ashworth VE, Castro C, Roper MC, Rolshausen PE (2019) Assessment of Pierce’s disease susceptibility in Vitis vinifera cultivars with different pedigrees. Plant Pathol 68:1079–1087. https://doi.org/10.1111/ppa.13027
DiRienzo JA, Casanoves F, Balzarini M, Gonzalez I, Tablada M, Robledo, C.W. (2014) InfoStat version 2014. (http://www.infostat.com.ar). Accessed Sept and Oct 2021
Feechan A, Anderson C, Torregrosa L, Jermakow A, Mestre P, Wiedemann-Merdinoglu S, Merdinoglu D, Walker AR, Cadle-Davidson L, Reisch B, Aubourg S, Bentahar N, Shrestha B, Bouquet A, Adam-Blondon AF, Thomas MR, Dry IB (2013) Genetic dissection of a TIR-NB-LRR locus from the wild North American grapevine species Muscadinia rotundifolia identifies paralogous genes conferring resistance to major fungal and oomycete pathogens in cultivated grapevine. Plant J 76:661–674. https://doi.org/10.1111/tpj.12327
Foria S, Magris G, Morgante M, Di Gaspero G (2018) The genetic background modulates the intensity of Rpv3-dependent downy mildew resistance in grapevine. Plant Breed 137:220–228. https://doi.org/10.1111/pbr.12564
Gallois JL, Moury B, German-Retana S (2018) Role of the genetic background in resistance to plant viruses. Int J Mol Sci 19:2856. https://doi.org/10.3390/ijms19102856
Guilhabert MR, Kirkpatrick BC (2005) Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Mol Plant Microbe Interact 18:856–868. https://doi.org/10.1094/MPMI-18-0856
Harakava R, Martins EMF, Ikuno A, Ferreira VCA, Guzzo S (2001) Molecular studies of the endo-polygalacturonase gene from Xylella fastidiosa. Funct Genome Symp Semin 17:37–38
Hopkins DL (1980) Use of pin-prick inoculation technique demonstrates variability in virulence of Pierce’s disease bacterium. In: Proceedings of VIIth International Conference of Viruses Grapevines, 1980 pp. 177–180
Jamieson PA, Shan L, He P (2018) Plant cell surface molecular cypher: receptor-like proteins and their roles in immunity and development. Plant Sci 274:242–251. https://doi.org/10.1016/j.plantsci.2018.05.030
Jones L, Riaz S, Morales-Cruz A, Amrine KC, McGuire B, Gubler WD, Walker MA, Cantu D (2014) Adaptive genomic structural variation in the grape powdery mildew pathogen Erysiphe Necator. BMC Genomics 15:1081. https://doi.org/10.1186/1471-2164-15-1081
Kalunke RM, Tundo S, Benedetti M, Cervone F, De Lorenzo G, D’Ovidio R (2015) An update on polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein that protects crop plants against pathogens. Front Plant Sci 6:146. https://doi.org/10.3389/fpls.2015.00146
Killiny N, Almeida RP (2009) Host structural carbohydrate induces vector transmission of a bacterial plant pathogen. Proc Nat Acad Sci 106:22416–22420. https://doi.org/10.1073/pnas.0908562106
Krivanek AF, Walker MA (2005) Vitis resistance to Pierce’s disease is characterized by differential Xylella fastidiosa populations in stems and leaves. Phytopathology 95:44–52. https://doi.org/10.1094/PHYTO-95-0044
Krivanek AF, Famula TR, Tenscher A, Walker MA (2005a) Inheritance of resistance to Xylella fastidiosa within a Vitis rupestris x Vitis arizonica hybrid population. Theor Appl Genet 111:110–119. https://doi.org/10.1007/s00122-005-1999-3
Krivanek AF, Stevenson JF, Walker MA (2005b) Development and comparison of symptom indices for quantifying grapevine resistance to Pierce’s disease. Phytopathology 95:36–43. https://doi.org/10.1094/PHYTO-95-0036
Krivanek AF, Riaz S, Walker MA (2006) Identification and molecular mapping of PdR1, a primary resistance gene to Pierce’s disease in Vitis. Theor Appl Genet 112:1125–1131. https://doi.org/10.1007/s00122-006-0214-5
Lapin D, Van den Ackerveken G (2013) Susceptibility to plant disease: more than a failure of host immunity. Trends Plant Sci 18:546–554. https://doi.org/10.1016/j.tplants.2013.05.005
Lindow S, Newman K, Chatterjee S, Baccari C, Iavarone AT, Ionescu M (2014) Production of Xylella fastidiosa diffusible signal factor in transgenic grape causes pathogen confusion and reduction in severity of Pierce’s disease. Mol Plant Microbe Interact 27:244–254. https://doi.org/10.1094/MPMI-07-13-0197-FI
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mortensen JA (1968) The inheritance of resistance to Pierce’s disease in Vitis. J Am Soc Hortic Sci 92:331–337
Nascimento R, Gouran H, Chakraborty S, Gillespie HW, Almeida-Souza HO, Tu A, Rao BJ, Feldstein PA, Bruening G, Goulart LR, Dandekar AM (2016) Type II secreted lipase/esterase LesA is a key virulence factor required for Xylella fastidiosa pathogenesis in grapevines. Sci Rep 6:18598. https://doi.org/10.1038/srep18598
Newman KL, Almeida RP, Purcell AH, Lindow SE (2004) Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc Natl Acad Sci 101:1737–1742. https://doi.org/10.1073/pnas.0308399100
Rapicavoli JN, Blanco-Ulate B, Muszyński A, Figueroa-Banderas R, Morales-Cruz A, Azadi P, Dobruchowska JM, Castro C, Cantu D, Roper MC (2018) Lipopolysaccharide O-antigen delays plant innate immune recognition of Xylella fastidiosa. Nat Commun 9:390. https://doi.org/10.1038/s41467-018-02861-5
Riaz S, Krivanek AF, Xu K, Walker MA (2006) Refined mapping of the Pierce’s disease resistance locus, PdR1, and sex on an extended genetic map of Vitis rupestris × V. arizonica. Theor Appl Genet 113:1317–1329. https://doi.org/10.1007/s00122-006-0385-0
Riaz S, Tenscher AC, Rubin J, Graziani R, Pao SS, Walker MA (2008) Fine-scale genetic mapping of two Pierce’s disease resistance loci and a major segregation distortion region on chromosome 14 in grape. Theor Appl Genet 117:671–681. https://doi.org/10.1007/s00122-008-0802-7
Riaz S, Tenscher AC, Graziani R, Pao SS, Krivanek AF, Ramming DW, Walker MA (2009) Using marker-assisted selection to breed Pierce’s disease-resistant grapes. Am J Enol Vitic 60:199–207
Riaz S, Huerta-Acosta K, Tenscher AC, Walker MA (2018) Genetic characterization of Vitis germplasm collected from the southwestern US and Mexico to expedite Pierce’s disease-resistance breeding. Theor Appl Genet 131:1589–1602. https://doi.org/10.1007/s00122-018-3100-z
Riaz S, Tenscher A, Hu R, Romero N, Ng D, Walker AM (2019) Genetic mapping of Pierce’s disease resistance in germplasm collected from the Southwestern US and Mexico. In: 70th ASEV National Conference, Napa, California
Riaz S, Tenscher AC, Heinitz CC, Huerta-Acosta KG, Walker MA (2020) Genetic analysis reveals an east-west divide within North American Vitis species that mirrors their resistance to Pierce’s disease. PLoS ONE 15(12):e0243445. https://doi.org/10.1371/journal.pone.0243445
Roper MC, Greve LC, Warren JG, Labavitch JM, Kirkpatrick BC (2007) Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol Plant Microbe Interact 20:411–419. https://doi.org/10.1094/MPMI−20-4-041
Silva CJ, van den Abeele C, Ortega-Salazar I, Papin V, Adaskaveg JA, Wang D, Casteel CL, Seymour GB, Blanco-Ulate B (2021) Host susceptibility factors render ripe tomato fruit vulnerable to fungal disease despite active immune responses. J Exp Bot 72:2696–2709. https://doi.org/10.1093/jxb/eraa601
Sun Q, Sun Y, Walker MA, Labavitch JM (2013) Vascular occlusions in grapevines with Pierce’s disease make disease symptom development worse. Plant Physiol 161:1529–1541. https://doi.org/10.1104/pp.112.208157
Varela LG, Smith RJ, Phillips PA (2001) Pierce’s disease. Agricultural and Natural Resources Publication University of California, Oakland
Walker MA, Riaz S (2013) Genetic mapping of Xylella fastidiosa resistance gene(s) in grape germplasm from the Southern United States. In: Proceedings of the 2013 Pierce’s Disease Research Symposium, Sacramento, California
Walker MA, Riaz S (2014) Map-based identification and positional cloning of Xylella fastidiosa resistance genes from known sources of Pierce’s disease resistance in grape. In: Proceedings of the 2014 Pierce’s Disease Research Symposium, Sacramento, California
Walker MA, Cantu D, Riaz S, Aguero C (2015) Molecular breeding support for the development of Pierce’s disease resistant winegrapes. In: Proceedings of the 2015 Pierce’s Disease Research Symposium, Sacramento, California
Walker MA, Tenscher AC, Riaz S, Romero N (2021) Grapevine plant named ‘Camminare noir’. (US Patent No: PP32929); ‘Paseante noir’ (US Patent No: PP33039); ‘Errante noir’ (US Patent No PP32999); ‘Caminante blanc’ (US Patent No PP33015); ‘Ambulo blanc’ (US Patent No PP32949)
Zaini PA, Nascimento R, Gouran H, Cantu D, Chakraborty S, Phu M, Goulart LR, Dandekar AM (2018) Molecular profiling of Pierce’s disease outlines the response circuitry of Vitis vinifera to Xylella fastidiosa infection. Front Plant Sci 9:771. https://doi.org/10.3389/fpls.2018.00771
Acknowledgements
We are grateful for the technical assistance of Rong Hu, Dan Ng and Ninfa Romero. We also thank Dr. Ian Dry for kindly providing plasmid pCLB2301NK.
Funding
This study was funded by the California Department of Food and Agriculture PD/GWSS Board.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CBA, SR and ACT. The first draft of the manuscript was written by CBA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interests.
Additional information
Communicated by Klaus Eimert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Agüero, C.B., Riaz, S., Tenscher, A.C. et al. Molecular and functional characterization of two RGA type genes in the PdR1b locus for Pierce’s disease resistance in Vitis arizonica/candicans. Plant Cell Tiss Organ Cult 151, 497–510 (2022). https://doi.org/10.1007/s11240-022-02366-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02366-6