Abstract
Douglas fir (Pseudotsuga menziesii) is one of Europe’s most important non-native tree species due to its drought tolerance as well as timber quality and yield. To obtain superior seed from selected parental trees, breeding programs had been established in seed orchards. Douglas fir seed is used as source material for somatic embryogenesis with the aim to select elite genotypes invaluable for clonal mass propagation. To improve given protocols for somatic embryo initiation, we used immature Douglas fir zygotic embryos as explants and abscisic acid (ABA) as plant growth regulator in contrast to the application of auxins and cytokinins. With ABA supplementation, induction frequencies were slightly but in mean higher than with auxin/cytokinin, showing also a strong genotype effect. This offered the possibility to capture SE cultures from otherwise recalcitrant crosses. Furthermore, we observed remarkable differences between the two sets of plant growth regulators concerning the morphological development of the explants, including the absence of non-embryogenic callus by using ABA as inducer. This simplifies the detection of events and the handling of the obtained cultures. Nevertheless, a histological approach suggested, that the same competent cells are addressed by the different hormonal stimulation. Besides, we studied the influence of different points in time of cone harvest, two different basal media and different genetic backgrounds of the explants as well as the maturation ability of the induced embryogenic cultures. In sum, we were able to improve the first steps of somatic embryogenesis and to maintain a significantly higher number of high-value genotypes.
Key message
The use of Abscisic acid as plant growth regulator for initiation of somatic embryogenesis and application of activated charcoal for callus management during maintenance increased genotype capture in Douglas fir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Douglas fir (Pseudotsuga menziesii [MIRB.] FRANCO) is one of the major, non-native tree species used for forestry in Western Europe, due to its productivity and ecological performance. Especially its superior tolerance to drought stress in comparison to the native Picea abies is a desired property with respect to climate changes and global warming. On the other hand, epidemic appearance of pests (esp. Rhabdocline pseudotsugae and Phaeocryptopus gaeumannii) and sensitivity towards late frosts of some proveniences are limiting factors for Douglas fir plantations esp. in Germany (Konnert 2009). For effective afforestation, the usage of certified and superior material from breeding programs is essential and demanded by the European Union (directive 1999/105/EC). Seed orchards and direct crosses of selected parental trees deliver certified seeds, but are vulnerable to pest outbreaks (esp. insects) and environmental impacts (e.g. summer droughts). Furthermore, the trees sufficiently flourish only in an interval of several years.
Clonal propagation of elite genotypes is a possibility to gain superior plant material – independent of seed availability. Most conifers lack the possibility to produce cuttings, therefore somatic embryogenesis (SE) is seen as the most effective propagation method for nearly all conifers in large scale (e.g. Lelu-walter et al. 2013). The process of SE includes several consecutive steps: First, SE is induced in vitro on a somatic cell of an explant, followed by multiplication of the embryogenic culture (EC), maturation of the somatic embryos and conversation into plantlets.
Although SE is a promising tool for the vegetative propagation of conifers, the method still has some challenges to overcome. As it is (yet) not possible to induce SE on adult trees (see e.g. Bonga 2017 for summary), the prerequisite for a successful application of the whole biotechnological process is the establishment of reliable protocols to induce and maintain SE from zygotic embryos (ZE). A lot of research has already been conducted in developing optimized compositions of culture media, sterilization and preparation methods as well as culture regimes (e.g. Pullman & Bucalo 2014; Ramírez-Mosqueda et al. 2019; Arrillaga et al. 2019 etc.). Especially the recalcitrance of certain crossings or genotypes remains a problem in many conifer species (Bonga et al. 2010), as this restricts the genetic resources.
In general, somatic embryogenesis is seen as a cellular reaction to stress or to a local change of the internal auxin concentration. Presumably, external stimuli (especially PGRs) trigger the de novo synthesis or the relocalisation of auxin (Feher 2015). In this context, 2,4-D is one of the widely used and the most effective elicitor of SE, as it either acts directly as an auxin or/and activates stress related genes.
Available protocols for the initiation of SE on Douglas fir claim the usage of culture media with cytokinins (CYTs) and auxins (AUXs) as plant growth regulators (PGRs), sometimes in combination with activated charcoal (AC; e.g. Pullman et al. 2003; Reeves et al. 2018). Following this approach, initiation percentages often strongly depend on the genotype. For example, Pullman et al. (2009) mentioned initiation percentages for Douglas fir on immature ZEs from controlled crosses between 5.6 and 55.5%. In addition, induced cultures often contain non-embryogenic callus (NEC) and therefore show a poor rate of growth and need special care and handling, as reported in Gautier et al. (2016) and Reeves et al. (2018).
In general, callus is defined as a mass of cells, growing unorganized and often exclusively under the influence of PGRs. For conifers, the regeneration of organs or plants out of callus cells is not (yet) possible, reflecting the high recalcitrance of these taxa towards growth stimulating conditions (Bonga et al. 2010). Hence, during the cultivation of EC the growth of callus is undesired. As NEC grows quickly and is sometimes hardly distinguishable from EC, it can cause the loss of embryogenic capability of the whole culture.
To overcome these problems, we aimed to forego auxins (as very effective promotors of callus growth). Instead, we used ABA as sole PGR in the induction process, as also described in Aitken-Christie & Parkes (1996) for Pinus radiata and in Nishiwaki et al. (2000) for Daucus carota.
Usually, in relation to SE, ABA is used to promote the transition from proliferation to maturation (Rai et al. 2011). Furthermore, ABA is responsible for the development of the desiccation tolerance of the embryo and prevents its precocious germination. To produce conifer somatic embryos, the addition of ABA is a standard mean to synchronise the maturation of embryos as well as improving their quality and is therefore used in concentrations of 20–170 µM.
However, in the last decades, it has also been applied to optimize the initiation of SE for several conifer species, by using it as an additional agent to the conventional culture media with cytokinins and auxins and in concentrations of circa 3,7 µM (e.g. for Loblolly pine: Pullman et al. 2003, for Fraser fir: Pullman et al. 2016). For Douglas fir, Pullman et al. showed the benefit of this concentration and raised the induction efficiency from 31.5 to 41% in 2009.
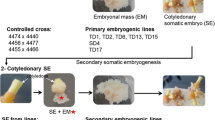
In our study, we used immature ZEs to induce SE on Douglas fir and compared ABA as an inducing agent with a combination of auxins and cytokinins in special regard to the reduction of NEC. In addition to that, we performed an investigation with mature somatic embryos to validate our data and to execute histologic analysis. We further suppressed subsequent growth of NEC by adding AC to the proliferation medium (Table 4). The further development of differently induced somatic embryos was investigated. Additionally, other relevant parameters of SE initiation were analyzed: the developmental stage of the ZE (explant), the influence of the genetic background of the explant and the basal medium (GLITZ from Reeves et al. 2018 and BM from Gupta et al. 1995).
Materials and methods
Immature cones from Douglas fir (Pseudotsuga menziesii) were collected from seed orchards (directed crosses) in Saxony, Germany. This was carried out weekly at specific points of time, which were defined before, based on developmental observations (classification of the specific developmental stages: see Table 3). After harvest, cones were kept at 2 °C until preparation. Seeds were separated and sterilized (3 min in 0.2% HgCl2). The seed coat was removed under aseptic conditions. For induction of SE in 2011–2016, the apical parts of the megagametophytes (MGs) were cut off so that the ZEs protrude (Fig. 1B). For the experiments in 2018, ZEs were isolated from the MGs.
Induction and characteristics of ZEs and ECs of P. menziesii: A–C ZEs in successive developmental stages (classification, see Table 3): C—stage 1; D—stage 2; E—stage 3; D–G formation of somatic embryos on ZEs, after six weeks on A BM_ABA, somatic embryos emerging on the hypocotyl; B BM_CYT/AUX, C Glitz_ABA, D Glitz_CYT/AUX; H freshly initiated somatic embryos without NEC formed on BM_ABA; I freshly initiated “mixed culture” of somatic embryos (arrow 1) and NEC (arrow 2) formed on BM_CYT/AUX
Three explants, respectively, were placed onto the induction medium (Table 4) in petri dishes (60 mm diameter).To induce secondary somatic embryogenesis, 50 mature somatic embryos (five per petri dish) of three genotypes of different crosses were transferred to Glitz_CYT/AUX and Glitz_ABA respectively. All petri dishes were sealed with parafilm and cultured in darkness at 23 °C for 8 weeks. Initiated tissue was analyzed with a light microscope to determine the presence of somatic embryos or NEC. Subsequently, somatic embryos were transferred to multiplication medium (Table 4).
For the induction experiments two different basal media were used: BM (modified after Gupta et al. 1995) for the experiments in 2011 and 2013–2016, and Glitz (modified after Reeves et al. 2018) for the experiments in 2018 and 2019. Each basal induction medium was supplemented with either a combination of cytokinins and auxins (BM_CYT/AUX and Glitz_CYT/AUX) or with ABA solely (BM_ABA and Glitz_ABA, see Table 4). For most experiments, the explants were distributed equally to both media types (cf. Table 1).
Table 1 shows the sampling and explant origin (cross) (Tables 5 and 6). In 2016, an additional sample of 680 explants from two crosses (AxF and AxE) was used to investigate the difference between two ABA-concentrations (40 and 100 µM). However, no statistical differences in the initiation frequency could be observed (Chi square = 0.103; df = 1; p = 0,748), so both concentrations were summarized for the analyses.
For experiments to optimize culture of Douglas fir ECs, basal medium BM (see Table 4) was used as control. Further, we used BM2 without PGRs and BM2 supplemented with 1 g/l activated charcoal (Duchefa Biochemie B.V, Haarlem, NL).
For further investigations, mature embryos and plantlets of Douglas fir were produced, using an adapted protocol of Kraft and Kadolsky (2018).
For histological analysis, mature somatic embryos, cultured on Glitz_CYT/AUX or Glitz_ABA at 23 °C at darkness, were sampled at days 0, 10 and 15. Single embryos were fixed for 24 h in AFE (Table 2), subsequently washed with 3% salt-buffered saline (PBS), drained stepwise with a rising concentration of ethanol and were afterwards embedded in paraffin. Sections (10 µm) were stained with alcian blue in combination with periodic acid Schiff reaction and a counterstain with hematoxylin.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistic 24. To compare PGR treatments and data of several years and crosses, absolute frequency of SE occurrence was analyzed with Chi-square independence tests (Fig. 4, Tables 5 and 6). To determine the influence of the developmental stage and the genetic background of the explant, the data set from the experiments of 2011–2016 was used, performing a logistic regression. For comparison of maturation ability of the somatic embryos induced on different medium types, a two-sided t-test was carried out.
Results
ABA as an inducer of SE on Douglas fir
The use of ABA led overall to a significantly higher induction frequency of SE on ZEs of Douglas fir than under standard conditions, using cytokinin/auxin stimulation (Chi square = 18.6; df = 1; p < 0.01, ϕ = 0.05), independent of the tested concentration (40 and 100 µM, data not shown). Thus, it was possible to induce SE on seven out of eight tested crosses. The induction efficiency was genotype-specific and dependent on further external factors (see below).
The macroscopic analysis of ZEs as explants for ABA-induced SE revealed an elongation of their hypocotyl and even subsequent germination—especially when explants of higher developmental stages were used (Fig. 1D). Somatic embryo formation usually started after five weeks in culture at the hypocotyl. A histological analysis of mature somatic embryos as explants for induction of secondary somatic embryogenesis gave insights to the developmental differences caused by CYT/AUX application compared to ABA (Figs. 2 and 3). Treatment with a combination of cytokinin and auxin caused massive cell divisions of epidermal and subepidermal cells of the basal hypocotyl within 5 days (Fig. 2C and D). After 10 d, tissue structure in this region vanished, replaced by sections with loose aggregation of vacuolated cells and clusters of small dividing cells (Fig. 2E and G). In contrast, tissues of explants treated with ABA remained highly organized. After 15 days, parenchymatic cells around the provascular tissue showed increased cell divisions, as the whole embryo started to grow. In addition, subepidermal cells underwent cell division and caused ruptures of the epidermal cell layer. These spots became “protuberances”, containing clusters of dividing cells and bigger, vacuolated cells (Fig. 3E) and later gave rise to the newly built embryos.
Histologic and morphologic reactions of SE initiation conditions on P. menziesii somatic embryos with CYT/AUX stimulation (basal media Glitz); A + B control without hormonal stimulus; A transverse section of the hypocotyl; B top view of a mature somatic embryo; C + D explants after 5d of culture; C transverse section of the basal hypocotyl reveals increased cell division of epidermal and subepidermal cells; D morphological reactions start with disorganization of the structure of the basal hypocotyl; E–G explants after 10d of culture; C transverse section of the hypocotyl reveals areas with loose cell cohesion (arrow 1) and clusters of dividing cells (arrow 2); F top view; G transverse section of the hypocotyl (detail view) with a cluster of dividing cells (circle); H explant after 30d of culture: the hypocotyl section turned into an aggregation of dedifferentiated cells. (Color figure online)
histologic and morphologic characteristics of SE initiation conditions on P. menziesii somatic embryos with ABA stimulation (basal media Glitz); A + B explants after 10d of culture; A transverse section of the basal hypocotyl, pinkish color indicates polysaccharides; B morphological reactions start with growth of the hole embryo; C–E explants after 15d of culture; C: transverse section of the basal hypocotyl, the epidermis is disrupted by protuberances (arrow); D top view of an embryo with protuberances at the hypocotyl (arrow); E detailed view of C (red box), protuberances enclose vacuolated cells (arrow) and clusters of dividing cells (circle); F + G explant after 40d of culture; F the somatic embryo has turned into a seedling, secondary somatic embryos emerge on the basal hypocotyl; G detailed view of F (red box). (Color figure online)
The genetic background of the ZEs (starting material) had a significant influence on the initiation frequency (see Table 5). One cross (FxH) did not develop any somatic embryos on basal media BM, independent of the used PGR. Furthermore, explants of different crosses showed different, but in tendency constant reactions towards ABA or CYT/AUX (Fig. 4A and B). For instance, progenies of cross AxF repeatedly showed the same reaction in all years tested: remarkably higher initiation percentages on medium containing ABA. Explants of the cross FxI showed the same tendency. In contrast, progenies of cross BxE showed higher output upon CYT/AUX treatment. These findings were confirmed by using mature somatic embryos as explants for secondary SE: For two genotypes, treatment with CYT/AUX was more efficient, whereas for the third genotype ABA treatment raised the induction frequency from 4% (CYT/AUX) to 78% (see Table 6).
In addition, the genotype of the respective parental trees (mother as well as father trees) had also a significant influence on the embryogenic ability of the explant. Mother trees A and D led to a significant rise of likelihood towards the average; mother tree B, F and I significantly lowered the likelihood. Regarding the father trees, four of six genotypes showed a significant influence: Trees E, F and I raised the likelihood of an explant to produce embryogenic tissue, whereas tree H clearly lowered the likelihood.
Induction frequency was also significantly influenced by the basal medium. On basal medium BM, induction efficiency with ABA-stimulation was in mean at 5.4%, with a maximum of 21.1% (explants from cross DxE). In comparison, a combination of cytokinins and auxins led to an induction efficiency of in mean 2.1%, with a maximum of 4.2% (explants from cross AxF, Table 5). On basal medium Glitz, induction efficiency for ZEs as explants was in mean at 46.6% with ABA-stimulation and 36.6% using the cytokinin/auxin-combination (Fig. 4B). The high effectiveness of Glitz was confirmed by using mature somatic embryos of three different genotypes as induction material. Induction efficiency with these explants was in mean at 39.6% with ABA-stimulation and 43,3% using the cytokinin/auxin-combination (Fig. 4B and Table 6).
The developmental stage of the ZEs as starting material for SE initiation showed a high impact on the induction frequency—regardless of the exogenously applied hormonal stimulus: SE mainly took place at ZEs of stage 3 (in mean 4.2%). The use of earlier stages led to lower frequencies (stage 1: 2.7% and stage 2: 3%). Almost no initiation of SE (0.3%) was detected for ZEs at stage 4 (Fig. 4C).
Induction of SE on P. menziesii and cultivation of initiated tissue, regarding the hormonal stimulus (CYT/AUX and ABA): A SE induction frequency in regard to hormonal stimulus per cross, basal medium BM (data from 2011 to 2016), significant differences between the hormonal stimuli are marked with asterisks (Chi square independence test); B initiation frequencies of SE for zygotic and somatic embryos as explant on basal medium Glitz (data from 2018 and 2019), significant differences between the hormonal stimuli are marked with asterisks; C initiation of SE (left Y-axis) and NEC (right Y-axis) depending on the developmental stage of the explants (ZEs, data 2011–2016); D Stability of initiated ECs (> 5 subcultures). The loss of ECs may be prevented by the use of BM2 + AC during subculture (− AC cultivation: culture on BM2 after initiation, + AC cultivation: additional subculture(s) on BM2 + AC
The influence of the PGR on the growth of NEC
In Douglas fir the formation of SE so far is frequently accompanied with growth of NEC, consisting of small and roundish cells (‘mixed culture’, Figs. 1I and 5A). Explants cultured on CYT/AUX containing media often showed only growth of NEC (20.9% of all explants on basal medium BM, 50.3% of ZE-explants on basal medium Glitz). The use of ABA as an inducer of SE in Douglas fir minimized the formation of NEC in a dose dependent manner (0% on 40 µM and 0.9% of all explants on 100 µM ABA). Initiated SE cultures did not show any additional non-embryogenic cells (Fig. 1H).
SE-steps of P. menziesii: A + B multiplication of “mixed cultures” of somatic embryos and NEC, 3 weeks after subculture on: A: multiplication medium BM2, B multiplication medium BM2 + 1 g/l AC, arrow 1: somatic embryos, arrow 2: NEC; C–E maturation, conversion and acclimatization of ABA-induced plant material; C mature somatic embryos; D plantlets, 4 weeks after conversion; E plantlets, 7 weeks after pricking
The percentage of NEC initiation clearly depended on the genotype (data not shown). The frequency of NEC formation on BM_CYT/AUX varied between 7 and 46% per cross, showing no correlation to the induction frequency of SE. In addition, the developmental stage of the ZEs showed a strong influence on NEC formation (Fig. 4C): On BM_CYT/AUX, NEC production increased significantly with the developmental stage of the ZEs up to 68% (stage 4). In contrast, the production of NEC on BM_ABA remained low (< 1%) when using ZEs of stages 1–3 and raised slightly on embryos of stage 4 (2.8%).
Culture and characteristics of ECs of Douglas fir
Once initiated, ECs were transferred to multiplication medium (see Table 4). In 2011, 2013 and 2014, 74% of CYT/AUX-initiated ECs and 83% of ABA-initiated ECs got lost during subculturing because of NEC growth (Fig. 4D: − AC cultivation). Test series to reduce this portion included cultivation of NEC and EC on BM2 without PGRs and BM2 + 1 g/l AC. By using the latter, NEC growth (increase of fresh weight (FW)) was reduced to 4–12% (depending on genotype) in comparison with the control (BM2 without AC) after 26 days. Complete lack of PGRs reduced NEC growth likewise, but to a lower extend (36% of FW increase of the control). In contrast, no reduction of FW growth was detectable for EC—neither on AC-containing nor on PGR-free medium. Subculture of freshly induced mixed cultures (NEC + EC) on AC-containing medium was therefore used to rescue somatic embryos: 4–5 weeks after AC treatment, NEC became brown, finally died and somatic embryos could easily be separated (Fig. 5B). AC treatment was repeated, if necessary. With this method, the loss of ECs due to overgrowth with NEC could significantly be reduced: for CYT/AUX-induced ECs to 43.4% and for ABA-induced ECs to 51.4% (Fig. 4D + AC cultivation).
The developmental ability of the somatic embryos (maturation and germination) was not significantly affected by the initiation medium (exemplarily shown in Fig. 5C–E). Well-developed mature embryos (WDE) of 18 genotypes induced on BM_CYT/AUX, and 42 genotypes initiated on BM_ABA were quantified. Both groups showed remarkable differences in morphology and yield of WDE. On average 620 WDE per g fresh weight were detected for the ABA-group and 949 WDE per g fresh weight for the CYT/AUX-group, but with very high standard deviations, respectively (sABA: 762.6; sCYT/AUX: 1142.1). Both groups contained genotypes with very high numbers of WDE (max. ABA: 2900 WDE/g FW, max. CYT/AUX: 3750 WDE/g FW) and genotypes, which did not produce any WDE at all. The difference between the arithmetical means of both groups was not significant (p = 0,194).
Discussion
ABA as an inducer of SE on Douglas fir
In our study, we showed that ABA induces SE on immature ZEs and mature somatic embryos of Douglas fir. More precisely, ABA primarily led to a further development of the Douglas fir ZEs used as explants, similar to the embryos’ natural development within the seeds. Numerous ZEs (on Glitz_ABA: ca. 1/3) showed germination, not affecting the ability to form somatic embryos (Fig. 1D). Under ABA stimulus, somatic embryos were formed on the basal part of the hypocotyl without any appearance of NEC. The same observation was made by Nishiwaki et al. (2000): SE was induced on seedlings of Daucus carota by using ABA directly out of epidermal cells located at the hypocotyl. In contrast, treatment with 2,4-D led to formation of somatic embryos out of provascular cells, after building a mass of small dividing cells around the central vascular elements (Guzzo et al. 1995). Nichiwalki et al. concluded, that the tissue, from which somatic embryos arose, differed—depending on the type of PGR.
Our histological analyses of Douglas fir somatic embryos revealed, that ABA treatment does not cause the epidermal cells to form somatic embryos. Instead, subepidermal cells undergo increased cell divisions. As CYT/AUX supplement also led to cell divisions in this cell layer, it is possible that ABA and CYT/AUX treatment affect the same competent cells to form somatic embryos. In general, the area between hypocotyl and root is presumed to be more competent for dedifferentiating processes (Bonga et al. 2010). Presumably, this effect is a result of the change of the local/cellular auxin level, as ABA itself in general has no stimulative impact on cell division. However, numerous studies showed that there is a link between the signal pathways of both hormones: for instance, several auxin response factors are inducible by ABA (Sun and Li 2014). Further ABA, amongst others, is responsible for the local biosynthesis and the polar transport of auxin during SE of Arabidopsis thaliana (Su et al. 2013). ABA is also known to be a critical messenger for the internal cell reaction towards stress. In this context, numerous studies prove the beneficial effect of an ABA addition within stress mediated SE initiation (i.e. Akula et al. 2000, Kikuchi et al. 2006).
It has also been proven for conifers, that ABA increases the frequency of SE induction when used as a supplement to “classical” media including auxin and cytokinin (e.g. Pullman et al. 2003 for Pinus taeda, Pullman et al. 2009 for Douglas fir). In those studies concentrations of 3.7 µM were used. However, higher doses of ABA in combination with auxins and cytokinins seem to have negative effects: Find et al. (2014) observed a lower induction frequency for Pinus radiata, when using 56 µM ABA as supplement. In own investigations with Douglas fir ZEs as explants, a medium composition with auxins, cytokinin and 40 µM ABA failed to induce any reaction at all (data not shown).
For conifers, the use of ABA as an inducing agent without any other PGR has so far only been described by Aitken-Christie & Parkes (1996) as a patent for Pinus radiata. In their publication (WO 1,996,037,096 A1) the authors described a 10% higher percentage of SE by using ABA in contrast to an auxin/cytokinin combination on average.
Our study on Douglas fir ZEs also showed a distinct benefit of using ABA to induce SE in contrast to the commonly used auxin/cytokinin combination. On average the initiation efficiency with ABA stimulation was 10% higher when using the basal medium Glitz and 3.3% higher when using BM. Considering no SE takes place on ZEs without any application of PGRs (data not shown), it is clearly shown that ABA can initiate the formation of SE on Douglas fir and therefore can be used as an alternative to the typically used auxin/cytokinin-combination. Especially, ABA treatment can potentially enable the capture of somatic embryos for otherwise recalcitrant genotypes, as we detected antagonistic reactions of some genotypes and crosses between the usage of ABA or CYT/AUX. Indeed, some of our crosses showed a much higher induction frequency with the ABA-medium (e.g. cross E), and progenies of other crosses did not react at all (e.g. cross I). The induction series with mature somatic embryos confirms this assumption: For two of three genotypes treatment with CYT/AUX was sufficient and led to high induction frequencies. The third genotype only showed numerous SE events by treatment with ABA.
Aitken-Christie & Parkes (1996) already mentioned that the effect of their ABA-medium depended on the genetic background of the progenies, as the different crosses they used reacted in a various manner to both media variations. Specific hormone regimes and levels, depending on species, genetic background and age of an explant, may play an important role regarding the response towards ABA. Investigations on loblolly pine revealed as well, that different culture media may be better suited for different genotypes in respect of SE induction (MacKay et al. 2006).
Most notably, the induction of SE on Douglas fir ZEs by ABA stimulus has one big practical advantage: As ABA exclusively induces SE without the formation of callus, there is no need for additional screening of the induced tissue under the light microscope, saving time and reducing labour costs.
The effect of the PGR regarding NEC formation
The use of auxins and cytokinins for SE induction on Douglas fir frequently leads to growth of NEC. Reeves et al. (2018) reported that up to 42% of their freshly induced SE lines were interspersed with NEC. Lowering the concentration of 2,4-D in the induction medium reduced this portion.
In our study, the complete absence of auxin and cytokinin in the induction medium caused a massive reduction of NEC growth (medium variation ABA, Fig. 4C). Moreover, ECs induced with ABA never contained NEC—in contrast to EC induced with CYT/AUX (Fig. 1H and I). However, after transferring the EC to 2,4-D-containing proliferating medium, NEC spontaneously regrew. In general, for a lot of plant species and types of explants, 2,4-D is the most effective elicitor of callus growth (Bhojwani and Dantu 2013). Our research for an optimised proliferating medium for Douglas fir revealed that the reduction of cytokinin alone has no effect on the growth of NEC, but presumably, 2,4-D solely causes the parallel growth of NEC and EC.
Noticeably, the growth of NEC on ZEs of Douglas fir was highly dependent on the developmental stage of the explants (medium variation BM_CYT/AUX), varying from 7.3% in stage 1 to 68.3% in stage 4 (Fig. 4C). This indicates, that the competent cells responding with the growth of NEC to CYT/AUX-stimulation are probably within a higher developmental degree or in a more differentiated cell status. Our histologic approach on mature somatic embryos during secondary SE revealed, that epidermal and subepidermal cells undergo increased cell division shortly after application of CYT/AUX. Following this, Douglas fir ZEs of higher developmental degrees probably respond more often with the growth of callus due to their differentiating dermal tissue types. In contrast, experiments with Arabidopsis thaliana regarding callus induction indicate that auxin and cytokinin stimulation exclusively addresses adult stem cells near the vascular elements of different organ types (Feher 2015).
To suppress the spontaneous growth of NEC during the cultivation/proliferation of the ECs, the addition of 1 g/l AC to the multiplication medium was beneficial (Fig. 5A and B). AC cultivation not only reduced NEC growth more effectively than PGR-free cultivation, but also led to die off nearly all NEC cells within 4–5 weeks. Due to the adsorbing properties of charcoal, the availability of the the PGR’s molecules is limited. Therefore, it seems obvious, that NEC cells require the uptake of 2,4-D and BAP to divide, whereas somatic embryos of Douglas fir keep their vitality and the ability to divide for at least four weeks without the addition of PGRs. This suggests that Douglas fir ECs are able to produce internal auxins and cytokinins. For hybrid larch Jourdain et al. (1997) detected that EC had a higher internal concentration of 2,4-D, Indole-3-pyruvic acid and Indole-3-acetic acid as NEC. However, the ability to grow on PGR free media seems to be species dependent, as some authors report about conifer EC, which show growth without PGR supplementation (e.g. Breton et al. 2005 for Pinus pinaster), whereas others detected the opposite (e.g. Bellarosa et al. 1992 for Picea abies).
Further parameters analysed with impact on SE of Douglas fir
The genetic background of the ZEs used as explants is one of the most important factors in regards to the induction frequency of SE in conifer species (see e.g. Cairney and Pullman 2007 for review). For Douglas fir Pullman et al. (2015) used seed from controlled pollination and observed considerable differences in the induction frequencies between the single crosses from 0.8 to 83% (values without arcsine √% transformation) when using the same medium (control 2301). Interestingly Reeves et al. (2018) reported more robust induction percentages (26–64%) between 5 half-sib families (open pollinated mother trees) of Douglas fir by using a modified Litvay-medium (GlitzB).
Our results also indicate a significant influence of the genotype to the likelihood to produce embryogenic cells. Depending on the respective cross, both, mother tree and pollen parent had a significant impact on the induction frequency of the progenies. For example, progenies of mother tree D showed significantly higher induction percentages than all other groups of progenies. For the pollen parents, tree H had the greatest impact, reducing the likelihood of its progenies to produce embryogenic cells significantly, representing an example of male inheritance. MacKay et al. (2006) indicated for Pinus teada that both mother tree and pollen parent can have significant effects on the initiation frequency. In summary, we observed big differences in regard to the SE initiation efficiency between our crosses despite a relatively low sample size of 9 trees. This supports the advice of MacKay et al. (2006) to carefully select mother trees, which increase SE induction frequencies to allow effective use of SE techniques.
In addition to that, our study validates the assumption, that the induction frequency of SE for Douglas fir depends on the developmental stage of the explants (ZEs), as already shown for many conifer species (see e.g. Isah 2016 for review). For both species immature ZEs have to be used, as mature embryos show a very low induction efficiency. As the immature ZEs undergo a genetic and ontogenetic development, it is not surprising that even for this material there are more or less suitable points in time for the induction of SE. Reeves et al. (2018) claimed the best point in time for induction of SE on Douglas-fir ZEs between the precotyledonary and early cotyledonary stage, which coincides with our findings. In contrast, Pullman et al. (2009) used ZEs within the bullet stage (early stage 2 according to our classification). However, it was clearly demonstrated that ZEs younger than the bullet-stage and older than the early cotyledonary stage were not suitable for initiation of SE in Douglas fir.
Another influencing factor is the composition of the basal medium. For our experiments with Douglas fir from 2011 until 2016, we used the basal medium BM described in Gupta et al. (1995) and observed max. 5% explants showing SE (control: CYT/AUX-supplementation). In contrast, Pullman et al. (2015) report an initiation efficiency of on average 44.5%, using the same basal medium (five crosses, media variation 2307 + 10 mg/l sodium dithionite). Reeves et al. (2018) used BM for Douglas fir as well and detected no initiation of SE at all. The preparation methods differed slightly (Pullman et al.: ZE with suspensor attached to the MG, Reeves et al.: ZE without MG, this study: ZE within the MG with apical part of the MG cut off), but the big differences suggest further (maybe laboratory specific) factors or significant differences in chemical compounds e.g. charcoal. Like Reeves et al. (2018) we found that the initiation efficiency raised remarkably, using Glitz as basal medium. On average 37% of all ZE explants and 43% of all mature SE explants showed an initiation of SE with standard PGR supplement (auxin + cytokinins), which is similar to the report of Reeves et al. (2018) where the induction efficiency was 41%.
The remarkable differences between the two basal media compositions on one hand, and the discrepancies between different laboratories despite using the same media formulation on the other, illustrate the sensitiveness of the process and the necessity for optimised and resilient protocols.
Initiation of SE on ABA-media does not affect the culture’s ability to produce mature embryos
As some studies predict a long-term effect regarding the characteristics and the developmental potential of the ECs caused by the initiation conditions (e.g. Garcia-Mendiguren et al. 2016), we were interested if the further development of ABA-induced ECs would be affected by this initiation treatment. The data from 2013, 2014 and 2016 indicates seriously problems with the transition of ABA derived cultures to the CYT/AUX containing multiplication medium, as ABA derived ECs were more often affected by growth of NEC. Applying the AC cultivation method inhibited this effect (Fig. 4D). The combined data of three trails (2015, 2016 and 2018) revealed no significant difference between ABA and CYT/AUX derived ECs (chi square = 1.62; df = 1; p = 0.2) Nevertheless, further investigations should be conducted to ensure, that ABA derived ECs do not have imbalanced internal hormone levels. Apart from that, our data do not show a significant difference between the two initiation treatments (ABA vs CYT/AUX) regarding the numbers of mature embryos produced. Differences in average embryo yields within the two groups were much higher than between them, and no different characteristics were detectable between the groups. Highly variable yields of WDE between several ECs of Douglas fir were also reported by Lelu-Walter et al. (2018). By using comparable maturation conditions, similar yields of WDE were detected.
In conclusion, our results clearly show, that ABA affects the developmental pattern of Douglas fir ZEs and can initiate SE. The usage of ABA as sole PGR lead to on average significantly more SE events for ZEs as explants, compared with the control group consisting of explants cultured on the standard PGR combination with auxins and cytokinins. Especially the combination with Glitz as basal medium was very effective, showing SE on in mean 47% of all explants. In addition, the use of ABA as the inducing agent avoids the growth of NEC, simplifying the screening, handling and maintenance of the initiated ECs. In combination with our improved subculture method using AC, we were able to raise the portion of captured EC for Douglas fir significantly. More analyses are necessary to find the underlying mechanisms of the initiating effect of ABA and to validate the data with ZEs on Glitz basal medium. Irrespective of that, the protocols described here can be used to improve the initiation of SE on Douglas fir, especially for otherwise recalcitrant genotypes or crosses. This allows addressing a wider range of genetic variation and thus preserving high valuable traits.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Abbreviations
- ABA:
-
Abscisic acid
- AC:
-
Activated charcoal
- AUX:
-
Auxin
- BAP:
-
6-Benzylaminopurine
- CYT:
-
Cytokinin
- EC:
-
Embryogenic culture
- FW:
-
Fresh weight
- NEC:
-
Non-embryogenic callus
- MG:
-
Megagametophyte
- PGR:
-
Plant growth regulator
- SE:
-
Somatic embryogenesis
- vs:
-
Versus
- WDE:
-
Well-developed embryo
- ZE:
-
Zygotic embryo
References
Aitken-Christie J, Parkes BD (1996) Improved embryogenesis process for initiation and maturation, PCT Pub No. WO 1996037096 A1, PCT publication date: 28 November 1996
Akula A, Akula C, Bateson M (2000) Betaine a novel candidate for rapid induction of somatic embryogenesis in tea (Camellia sinensis (L.) O. Kuntze). Plant Growth Regul 30:241–246. https://doi.org/10.1023/A:1006323213621
Arrillaga I, Morcillo M, Zanón I, Lario F, Segura J, Sales E (2019) New approaches to optimize somatic embryogenesis in maritime pine. Front Plant Sci 10:138. https://doi.org/10.3389/fpls.2019.00138
Bellarosa R, Mo LH, von Arnold S (1992) The influence of auxin and cytokinin on proliferation and morphology of somatic embryos of Picea abies (L.) Karst. Ann Bot 70:199–206. https://doi.org/10.1093/oxfordjournals.aob.a088460
Bhojwani SS, Dantu PK (2013) Plant tissue culture: An introductory text. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1026-9
Bonga JM (2017) Can explant choice help resolve recalcitrance problems in in vitro propagation, a problem still acute especially for adult conifers? Trees 31:781–789. https://doi.org/10.1007/s00468-016-1509-z
Bonga JM, Klimaszewska KK, von Aderkas P (2010) Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tiss Organ Cult 100:241–254. https://doi.org/10.1007/s11240-009-9647-2
Breton D, Harvengt L, Trontin J et al (2005) High subculture frequency, maltose-based and hormone-free medium sustained early development of somatic embryos in maritime pine. In Vitro Cell Dev Biol-Plant 41:494. https://doi.org/10.1079/IVP2005671
Cairney J, Pullman GS (2007) The cellular and molecular biology of conifer embryogenesis. New Phytologist–Transley Reviews 176:511–536. https://doi.org/10.1111/j.1469-8137.2007.02239.x
Feher A (2015) Somatic embryogenesis–Stress induced remodeling of plant cell fate. Biochemica et Biophysica Acta 1849(4):385–402. https://doi.org/10.1016/j.bbagrm.2014.07.005
Find JI, Hargreaves CL, Reeves CB (2014) Progress towards initiation of somatic embryogenesis from differentiated tissues of radiata pine (Pinus radiata D. Don) using cotyledonary embryos. In Vitro Cell Dev Biol-Plant 50:190–198. https://doi.org/10.1007/s11627-013-9581-1
García-Mendiguren O, Montalbán IA, Goicoa T et al (2016) Environmental conditions at the initial stages of Pinus radiata somatic embryogenesis affect the production of somatic embryos. Trees 30:949–958. https://doi.org/10.1007/s00468-015-1336-7
Gautier F, Eliášová K, Reeves C, Rodriguez LS, Teyssier C. et al. (2016): What is the best way to maintain embryogenic capacity of embryogenic lines initiated from Douglas-fir immature embryos?, 4e IUFRO Working Party 2.09.02 conference “Somatic embryogenesis and other vegetative propagation technologies. Development and application of vegetative propagation technologies in plantation forestry to cope with a changing climate and environment”, La Plata, Argentina. ⟨hal-01603097⟩
Gupta PK, Timmis R, Timmis KA, Carlson WC, Welty EDE (1995) Somatic Embryogenesis in Douglas-fir (Pseudotsuga Menziesii). In: Jain SM, Gupta PK, Newton RJ (eds) Somatic Embryogenesis in Woody Plants Forestry Sciences, vol 44–46. Springer, Dordrecht
Guzzo F, Baldan B, Levi M et al (1995) Early cellular events during induction of carrot explants with 2,4-D. Protoplasma 185:28–36. https://doi.org/10.1007/BF01272751
Isah T (2016) Induction of somatic embryogenesis in woody plants. Acta Physiol Plant 38:118. https://doi.org/10.1007/s11738-016-2134-6
Jourdain I, Lelu M, Label P (1997) Hormonal changes during growth of somatic embryogenic masses in hybrid larch. Plant Physiol Biochem 35:741–749
Kikuchi A, Sanuki N, Higashi K et al (2006) Abscisic acid and stress treatment are essential for the acquisition of embryogenic competence by carrot somatic cells. Planta 223:637–645. https://doi.org/10.1007/s00425-005-0114-y
Konnert M. (2009): Genetische Aspekte und Herkunftsfragen der Douglasie. In: Engel J. (eds): Die Douglasie im nordostdeutschen Tiefland - Chancen und Risiken im Klimawandel; Hrsg.: Landeskompetenzzentrum Forst Eberswalde (LFE), Eberswalder Forstliche Schriftenreihe Band 43
Kraft A, Kadolsky M (2018) Hybrid Larch (Larix × eurolepis Henry). In: Jain S, Gupta P (eds) Step wise protocols for somatic embryogenesis of important woody plants. Forestry sciences, vol 84. Springer, Cham
Lelu-Walter M, Thompson D, Harvengt L et al (2013) Somatic embryogenesis in forestry with a focus on Europe: state-of-the-art, benefits, challenges and future direction. Tree Genet Genomes 9:883–899. https://doi.org/10.1007/s11295-013-0620-1
Lelu-Walter M, Gautier F, Eliášová K et al (2018) High gellan gum concentration and secondary somatic embryogenesis: two key factors to improve somatic embryo development in Pseudotsuga menziesii [Mirb.]. Plant Cell Tiss Organ Cult 132:137–155. https://doi.org/10.1007/s11240-017-1318-0
MacKay JJ, Becwar MR, Park Y et al (2006) Genetic control of somatic embryogenesis initiation in loblolly pine and implications for breeding. Tree Genet Genomes 2:1–9. https://doi.org/10.1007/s11295-005-0020-2
Nishiwaki M, Fujino K, Koda Y et al (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759. https://doi.org/10.1007/s004250000387
Pullman GS, Namjoshi K, Zhang Y (2003) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation with abscisic acid and silver nitrate. Plant Cell Rep 22:85–95. https://doi.org/10.1007/s00299-003-0673-y
Pullman GS, Johnson S, Bucalo K (2009) Douglas fir embryogenic tissue initiation. Plant Cell Tiss Organ Cult 96:75. https://doi.org/10.1007/s11240-008-9462-1
Pullman GS, Bucalo K (2014) (2014): Pine somatic embryogenesis: analyses of seed tissue and medium to improve protocol development. New Forest 45:353–377. https://doi.org/10.1007/s11056-014-9407-y
Pullman GS, Zeng X, Copeland-Kamp B, Crockett J, Lucrezi J, May SW, Bucalo K (2015) Conifer somatic embryogenesis: improvements by supplementation of medium with oxidation–reduction agents. Tree Physiol 35(2):209–224. https://doi.org/10.1093/treephys/tpu117
Pullman GS, Olson K, Fischer T et al (2016) Fraser fir somatic embryogenesis: high frequency initiation, maintenance, embryo development, germination and cryopreservation. New Forest 47:453–480. https://doi.org/10.1007/s11056-016-9525-9
Rai MK, Shekhawat NS, Harish, et al (2011) The role of abscisic acid in plant tissue culture: a review of recent progress. Plant Cell Tiss Organ Cult 106:179–190. https://doi.org/10.1007/s11240-011-9923-9
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Armas-Silva AA et al (2019) Effect of the thin cell layer technique in the induction of somatic embryos in Pinus patula Schl. et Cham. J For Res 30:1535–1539. https://doi.org/10.1007/s11676-018-0663-0
Reeves C, Hargreaves C, Trontin J et al (2018) Simple and efficient protocols for the initiation and proliferation of embryogenic tissue of Douglas-fir. Trees 32:175–190. https://doi.org/10.1007/s00468-017-1622-7
Su YH, Su YX, Liu YG et al (2013) Abscisic acid is required for somatic embryo initiation through mediating spatial auxin response in Arabidopsis. Plant Growth Regul 69:167. https://doi.org/10.1007/s10725-012-9759-2
Sun J, Li C (2014) Cross Talk of Signaling Pathways Between ABA and Other Phytohormones. In D-P. Zhang (ed.), Abscisic Acid: Metabolism, Transport and Signaling, Dordrecht: Springer, ISBN 978-94-017-9423-7
Acknowledgements
We would like to thank Dr. Heino Wolf and his staff from Staatsbetrieb Sachsenforst for providing the plant material (immature seeds) and for the long-time close and pleasent collaboration.
Funding
Open Access funding enabled and organized by Projekt DEAL. Parts of this work were financially supported by the German Federal Ministry of Food and Agriculture (BMEL) by decision of the German Bundestag through the Fachagentur Nachwachsende Rohstoffe (FNR), Grant Number 22034914 and 22034814 as well as the German Federal Ministry of Education and Research (BMBF), Grant Number 31P6534.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Preparation of immature zygotic embryos and mature somatic embryos was performed by MW. Data collection and analysis were performed by MW. The histological analysis was performed by IW. Drafting and finalisation of the manuscript was carried out by MW. JR helped to draft and edit the manuscript. Initial conception was done by KZ. Supervision and revision was done by AR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research involved no human participants and/or animals.
Consent for publication
This study has not been published before is not under consideration for publication anywhere else. All authors consent to the publication of the manuscript in PCTOC.
Additional information
Communicated by M. Angeles Revilla.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walther, M., Wagner, I., Raschke, J. et al. Abscisic acid induces somatic embryogenesis and enables the capture of high-value genotypes in Douglas fir (Pseudotsuga menziesii [MIRB.] Franco). Plant Cell Tiss Organ Cult 148, 45–59 (2022). https://doi.org/10.1007/s11240-021-02159-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02159-3