Abstract
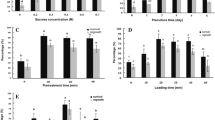
The present study described a droplet-vitrification cryopreservation for shoot tips of shallot (Allium cepa var. aggregatum), a small bulb onion. Shoot tips taken from in vitro stock shoots were precultured with 0.3 M and 0.5 M of sucrose, with 1 day for each concentration. Precultured shoot tips were treated with a loading solution containing 2 M glycerol and 0.6 M sucrose for 20 min and then exposed to plant vitrification solution 3 (PVS3) at 24 °C for 3 h of dehydration. Following exposure to PVS3, shoot tips were moved onto 5.0 µl PVS3 droplets on aluminum foil strips, followed by direct immersion into liquid nitrogen for 1 h. Frozen shoot tips were thawed by incubation in liquid MS medium containing 1.2 m sucrose for 20 min at room temperature, and then post-thaw cultured for shoot regrowth. Exposure of the shoot tips to PVS3 produced shoot regrowth (58%). Differential scanning calorimetry (DSC) detected 1.8% of freezable water in the shoot tips that had been dehydrated by PVS2, and no freezable water in those by PVS3 treatment. Exposure to PVS3 provided a broader safe temperature range (− 196 °C to − 88 °C), compared to that (− 196 °C to − 116 °C) of PVS2, for cryopreserved samples. Histological observations found that PVS3 dehydration allowed many cells in the apical dome and in the leaf primordia to survive following freezing in LN, while PVS2 dehydration resulted in much fewer surviving cells in the apical dome. The droplet-vitrification cryopreservation produced 56%, 72% and 32% shoot regrowth in cryopreserved shoot tips taken from in vitro shoots, adventitious buds regenerated from stem discs and field-grown bulbs, respectively. Advantages and disadvantages of the use of different source explants for cryopreservation were discussed. The droplet-vitrification cryopreservation produced 45% and 70% shoot regrowth in the additional two shallot genotypes ‘Kverve’ and ‘Lunteviga’. The results obtained in this study provide technical supports for setting-up cryo-bankings of genetic resources of shallots and other Allium species.
Key message
Establishment of a droplet-vitrification cryopreservation of shallot shoot tips provided technical supports for long-term preservation of diverse genetic resources and cryotherapy for virus eradication in shallot plants.





Similar content being viewed by others
Abbreviations
- ABIM:
-
Adventitious bud induction medium
- AD:
-
Apical dome
- 6-BA:
-
6-Benzylaminopurine
- CE:
-
Cryopreservation efficiency
- DSC:
-
Differential scanning calorimetry
- FAA:
-
Formalin-acetic acid-alcohol solution
- LN:
-
Liquid nitrogen
- LP:
-
Leaf primordium
- MS:
-
Murashige and Skoog (1962) medium
- NAA:
-
Naphthylacetic acid
- PVS:
-
Plant vitrification solution
- PVS2:
-
Plant vitrification solution 2
- PVS3:
-
Plant vitrification solution 3
- SCM:
-
Stock culture medium
- SE:
-
Standard error
- TB:
-
Toluidine blue
- TDZ:
-
Thidiazuron
References
Bettoni JC, Bonnart R, Shepherd A, Kretzschmar AA, Volk GM (2019) Cryopreservation of grapevine (Vitis spp.) shoot tips from growth chamber-sourced plants and histological observations. Vitis 58:71–78
Bi WL, Hao XY, Cui ZH, Pathirana R, Volk GM, Wang QC (2018) Shoot tip cryotherapy for efficient eradication of grapevine leafroll-associated virus-3 from diseased grapevine in vitro plants. Ann Appl Biol 173:261–270
Buitink J, Leprince O (2004) Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 48:215–228
Campos K, Diniz Y, Cataneo A, Faine L, Alves M, Novelli E (2003) Hypoglycaemic and antioxidant effects of onion, Allium cepa: dietary onion addition, antioxidant activity and hypoglycaemic effects on diabetic rats. Int J Food Sci Nutr 54:241–246
Chen H-Y, Liu J, Pan C, Yu J-W, Wang Q-C (2018) In vitro regeneration of adventitious buds from leaf explants and their subsequent cryopreservation in highbush blueberry. Plant Cell Tissue Organ Cult 134:193–204
Dobránszki J, Hudak I, Magyar-Tabori K, Jambor-Benczur E, Galli Z, Kiss E (2004) Effects of different cytokinins on the shoot regeneration from apple leaves of ‘Royal Gala’ and ‘M.26’. Int J Hortic Sci 101:69–75
Ellis D, Skogerboe D, Andre C, Hellier B, Volk G (2006) Implementation of garlic cryopreservation techniques in the national plant germplasm system. Cryo-Letters 27:99–106
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. Vitro Cell Dev Biol Plant 47:5–16
Fahy GM, MacFarlane D, Angell C, Meryman H (1984) Vitrification as an approach to cryopreservation. Cryobiology 21:407–426
Feng C-H, Cui Z-H, Li B-Q, Chen L, Ma Y-L, Zhao Y-H, Wang Q-C (2013) Duration of sucrose preculture is critical for shoot regrowth of in vitro-grown apple shoot-tips cryopreserved by encapsulation-dehydration. Plant Cell Tissue Organ Cult 112:369–378
Feng CH, Li BQ, Hu LY, Wang MR, Wang QC (2014) A comparison of two cryogenic protocols in cryopreservation of apple shoot tips. Acta Hortic 1039:161–166
Fritsch R, Friesen N (2002) Evolution, domestication and taxonomy. In: Rabinowitch HD, Currah L (eds) Allium crop science: recent advances. CABI Publishing, New York, pp 5–30
Harvengt L, Meier-Dinkel A, Dumas E, Collin E (2004) Establishment of a cryopreserved gene bank of European elms. Can J For Res 34:43–55
Keller ERJ (2005) Improvement of cryopreservation results in garlic using low temperature preculture and high-quality in vitro plantlets. Cryo-Letters 26:357–366
Kim H-H, Yoon J-W, Kim J-B, Engelmann F, Cho E-G (2005) Thermal analysis of garlic shoot tips during a vitrification procedure. Cryo-Letters 26:33–44
Kim H-H, Lee J-K, Yoon J-W, Ji J-J, Nam S-S, Hwang H-S, Cho E-G, Engelmann F (2006) Cryopreservation of garlic bulbil primordia by the droplet-vitrification procedure. Cryo-Letters 27:143–153
Kim HH, Lee JK, Hwang HS, Engelmann F (2007) Cryopreservation of garlic germplasm collections using the droplet-vitrification technique. Cryo-Letters 28:471–482
Kohmura H, Ikeda Y, Sakai A (1994) Cryopreservation of apical meristems of Japanese shallot (Allium wakegi A.) by vitrification and subsequent high plant regeneration. Cryo-Letters 15:287–298
Köpnick C, Grübe M, Stock J, Senula A, Mock H-P, Nagel M (2018) Changes of soluble sugars and ATP content during DMSO droplet freezing and PVS3 droplet vitrification of potato shoot tips. Cryobiology 85:79–86
Li B-Q, Feng C-H, Hu L-Y, Wang M-R, Chen L, Wang Q-C (2014) Shoot regeneration and cryopreservation of shoot tips of apple (Malus) by encapsulation–dehydration. Vitro Cell Dev Biol Plant 50:357–368
Li B-Q, Feng C-H, Wang M-R, Hu L-Y, Volk GM, Wang Q-C (2015) Recovery patterns, histological observations and genetic integrity in Malus shoot tips cryopreserved using droplet-vitrification and encapsulation-dehydration procedures. J Biotechnol 214:182–191
Li J-W, Ozudogru E-A, Li J, Wang M-R, Bi W-L, Lambardi M, Wang Q-C (2018) Cryobiotechnology of forest trees: recent advances and future prospects. Biodivers Conserv 27:795–814
Liu X-X, Wen Y-B, Cheng Z-H, Mou S-W (2017) Establishment of a garlic cryopreservation protocol for shoot apices from adventitious buds in vitro. Sci Hortic 226:10–18
Lynch PT, Souch GR, Zámeník J, Harding K (2016) Optimization of water content for the cryopreservation of Allium sativum in vitro cultures by encapsulation-dehydration. Cryo-Letters 37:308–317
Makowska Z, Keller J, Engelmann F (1999) Cryopreservation of apices isolated from garlic (Allium sativum L.) bulbils and cloves. Cryo-Letters 20:175–182
Mohammadi-Motlagh H-R, Mostafaie A, Mansouri K (2011) Anticancer and anti-inflammatory activities of shallot (Allium ascalonicum) extract. Arch Med Sci 7:38–44
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Niwata E (1995) Cryopreservation of apical meristems of garlic (Allium sativum L.) and high subsequent plant regeneration. Cryo-Letters 16:102–107
Pan C, Liu J, Bi W-L, Chen H-Y, Engelmann F, Wang Q-C (2018) Cryopreservation of small leaf squares-bearing adventitious buds of Lilium Oriental hybrid ‘Siberia’ by vitrification. Plant Cell Tissue Organ Cult 133:159–164
Rabinowitch HD, Currah L (2002) Allium crop science: recent advances. CABI Publishing, New York
Sakai WS (1973) Simple method for differential staining of paraffin embedded plant material using toluidine blue O. Stain Technol 48:247–249
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. Cryo-Letters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sestak J, Zamecnik J (2007) Can clustering of liquid water and thermal analysis be of assistance for better understanding of biological germplasm exposed to ultra-low temperatures? J Thermol Ana Calorim 88:411–416
Sittisart P, Yossan S, Prasertsan P (2017) Antifungal property of chili, shallot and garlic extracts against pathogenic fungi, Phomopsis spp., isolated from infected leaves of para rubber (Hevea brasiliensis Muell. Arg.). Agric Nat Resour 51:485–491
Teixeira AS, Faltus M, Zámečník J, González-Benito ME, Molina-García AD (2014) Glass transition and heat capacity behaviors of plant vitrification solutions. Thermochim Acta 593:43–49
Tubić L, Savić J, Mitić N, Milojević J, Janošević D, Budimir S, Zdravković-Korać S (2016) Cytokinins differentially affect regeneration, plant growth and antioxidative enzymes activity in chive (Allium schoenoprasum L.). Plant Cell Tissue Organ Cult 124:1–14
Volk GM, Walters C (2006) Plant vitrification solution 2 lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 52:48–61
Volk GM, Maness N, Rotindo K (2004) Cryopreservation of garlic (Allium sativum L.) using plant vitrification solution 2. Cryo-Letters 25:219–226
Volk GM, Bonnart R, Shepherd A, Yin ZF, Lee R, Polek M, Krueger R (2017) Citrus cryopreservation: viability of diverse taxa and histological observations. Plant Cell Tissue Organ Cult 128:327–334
Walters C (2004) Temperature dependency of molecular mobility in preserved seeds. Biophys J 86:1253–1258
Wang B, Li J-W, Zhang Z-B, Wang R-R, Ma Y-L, Blystad D-R, Keller EJ, Wang Q-C (2014) Three vitrification-based cryopreservation procedures cause different cryo-injuries to potato shoot tips while all maintain genetic integrity in regenerants. J Biotechnol 184:47–55
Wang M-R, Chen L, da Silva JAT, Volk GM, Wang Q-C (2018) Cryobiotechnology of apple (Malus spp.): development, progress and future prospects. Plant Cell Rep 37:689–709
Wang M-R, Zhang Z, Haugslien S, Sivertsen A, Rasmussen M, Wang Q-C, Blystad DR (2019) Cryopreservation of shallot (Allium cepa var. aggregatum) shoot tips by droplet-vitrification. Acta Hortic 1234:241–248
Wei H, Tye L, Bresnick E, Birt DF (1990) Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res 50:499–502
Yang J, Meyers KJ, van der Heide J, Liu RH (2004) Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J Agric Food Chem 52:6787–6793
Yin ZF, Bi W-L, Chen L, Zhao B, Volk GM, Wang Q-C (2014) An efficient, widely applicable cryopreservation of Lilium shoot tips by droplet vitrification. Acta Physiol Plant 36:1683–1692
Zhang J-M, Han L, Lu X-X, Volk GM, Xin X, Yin G-K, He J-J, Wang L, Chen X-L (2017) Cryopreservation of Jerusalem artichoke cultivars using an improved droplet-vitrification method. Plant Cell Tissue Organ Cult 128:577–587
Acknowledgements
We acknowledge financial supports from the Research Council of Norway (Project No. 255032/E50), NIBIO, and the Norwegian Genetic Resource Centre. Professional assistance obtained from Crop Research Institute, Czech Republic in the DSC test is appreciated as well. We also acknowledge the Image center of Norwegian University of Life Sciences for providing technical guidance and valuable courses for microscopy.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, MR., Zhang, Z., Zámečník, J. et al. Droplet-vitrification for shoot tip cryopreservation of shallot (Allium cepa var. aggregatum): effects of PVS3 and PVS2 on shoot regrowth. Plant Cell Tiss Organ Cult 140, 185–195 (2020). https://doi.org/10.1007/s11240-019-01721-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01721-4