Abstract
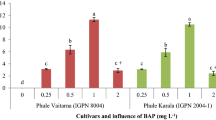
Morphogenic responses of two accessions of African yam bean to different concentrations of plant growth regulator supplements to Murashige and Skoog basal medium was investigated to develop a more efficient regeneration system. Mature embryo explants were cultured on growth regulator-free and BAP + NAA supplemented media. Nodal cuttings excised from 4-week old shoots of the regenerated embryos were cultured on media containing varying concentrations and combinations of 6-benzyl aminopurine (BAP), kinetin and α-naphthalene acetic acid (NAA). Growth regulator-free medium favored embryo regeneration and growth over supplemented media and both enhanced shoot regeneration and rooting, but could not induce multiple shoot formation on embryo explants. Multiple shoots were produced by nodal explants and the highest average number of shoots (5.3 ± 2.3), leaves (7.7 ± 3.6), roots (3.7 ± 2.9) and root length (3.1 ± 0.0 cm) were obtained on a medium with 0.6 mg l−1 BAP + 0.03 mg l−1 NAA for accession TSs154, while in TSs5, highest number of shoots (3.2 ± 2.5) and leaves (5.9 ± 1.5) were induced by 2.0 mg l−1 Kinetin + 0.05 mg l−1 NAA. Such differential morphogenic responses to culture media underline the genotypic control of in vitro propagation of this crop. Embryo and nodal explants rooted directly on shoot regeneration media, and regenerated plantlets were successfully acclimatized. The efficient regeneration system obtained will enhance genetic improvement of African yam bean by facilitating molecular genetic transformation for advanced breeding.




Similar content being viewed by others
References
Adesoye AI, Emese A, Olayode OM (2012) In vitro regeneration of African Yam Bean (Sphenostylis stenocarpa (Hochst ex. A. Rich.) Harms by direct organogenesis. Kasetsart J Nat Sci 46:592–602
Adewale BD (2011) Genetic diversity, stability and reproductive biology of African yam bean, Sphenostylis stenocarpa (Hochst. ex A. Rich.) Harms. PhD Thesis, University of Agriculture, Abeokuta
Adewale BD, Odoh NC (2013) A review on genetic resources, diversity and agronomy of African Yam Bean (Sphenostylis stenocarpa (Hochst. Ex A. Rich.) Harms): a potential future food crop. Sustain Agric Res 2(1):32–43. doi:10.5539/sar.v2n1p32
Akande SR, Balogun MO, Ogunbodede BA (2009) Effects of plant growth regulators and explant types on callus formation in African Yam Bean (Sphenostylis stenocarpa (Hochst. Ex A. Rich) Harms). Kasetsart J Nat Sci 43:442–448
Amoatey HM, Klu GYP, Bansa D, Kumaga FK, Aboagye LM, Benett SO, Gamedoagbao DK (2006) African yam bean (Sphenostylis stenocarpa) a neglected crop in Ghana. West Afr J Appl Ecol 1:53–60
Anwar F, Alghamdi SS, Ammar MH, Siddique KHM (2011) An efficient in vitro regeneration protocol for faba bean (Vicia faba L.). J Med Plants Res 5(28):6460–6467
Aremu AO, Bairu MW, Dolezˇal K, Finnie JF, Van Staden J (2012) Topolins: a panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult 108:1–16
Aremu AO, Plačková L, Bairu MW, Novák O, Plíhalová L, Doležal K, Finnie JF, Van Staden J (2014) How does exogenously applied cytokinin type affect growth and endogenous cytokinins in micropropagated Merwilla plumbea? Plant Cell Tissue Organ Cult 118:245–256. doi:10.1007/s11240-014-0477-5
Atif RM, Patat-Ochatt EM, Svabova L, Ondrej V, Klenoticova H, Jacas L, Griga M, Ochatt SJ (2013) Gene transfer in legumes. In: Lüttge U, Beyschlag W, Francis D, Cushman J (eds) Progress in botany, vol 74. Springer, Berlin, pp 37–100. doi:10.1007/978-3-642-30967-0-2
Avenido RA, Hattori K (2000) Benzyladenine-induced adventitious shoot regeneration rom hypocotyls of adzuki bean [Vigna angularis (Willd) Ohwi and Ohashi]. Plant Growth Regul 31:147–153
Barpete S, Khawar KM, Özcan S (2014) Differential competence for in vitro adventitious rooting of grass pea (Lathyrus sativus L.). Plant Cell Tissue Org Cult 119:39–50. doi:10.1007/s11240-014-0512-6
Brar MS, Al-Khayri JM, Morelock TE, Anderson DE (1999) Genotypic response of cowpea Vigna unguiculata (L.) to in vitro regeneration from cotyledon explants. In Vitro Cell Dev Biol Plant 35:8–12
Chandra A, Pentai D (2003) Regeneration and genetic transformation of grain legumes; an overview. Curr Sci 84:381–387
Chitra DSV, Padmaja G (2002) Seasonal influence on axillary bud sprouting and micropropagation of elite cultivars of mulberry. Sci Hortic 92:55–68
Devi P, Radha P, Sitamahalakshmi L, Syamala D, Kumar SM (2004) Plant regeneration via somatic embryogenesis in mung bean [Vigna radiata (L.) Wilczek]. Sci Hortic 99:1–8
Durieu P, Ochatt SJ (2000) Efficient intergeneric fusion of pea (Pisum sativum L.) and grass pea (Lathyrus sativus L.) protoplasts. J Exp Bot 51:1237–1242
Ekop AS (2006) Changes in Amino Acid Composition of African Yam Beans (Sphenostylis stenocarpas) and African Locust Beans (Parkia filicoida) on Cooking. Pak J Nutr 5(3): 254–256, 2006 ISSN 1680-5194 © Asian Network for Scientific Information
Fasoyiro SB, Ajibade SR, Omole AJ, Adeniyan ON, Farinde EO (2006) Proximate, minerals and antinutritional factors of some under-utilized grain legumes in south-western Nigeria. Nutr Food Sci 36(1):18–23
Gatica Arias AM, Valverde JM, Fonseca PR, Malera MV (2010) In vitro plant regeneration system for common bean (Phaseolus vulgaris): effect of N6-benzylaminopurine and adenine sulphate. Electron J Biotechnol 131:1–8. doi:10.2225/vol13-issue1-fulltext-7
Geetha N, Venkatachalam P, Prakash V, Sita GL (1998) High frequency induction of multiple shoots and plant regeneration from seedling “plants of pigeon pea (Cajanus cajan L.)”. Curr Sci 75:1036–1041
GRIN (Genetic Resources Information Network) (2009). GRIN taxonomy for plants npgs/html/. http://www.arsgrin.gov/cgibin/6. Accessed Feb 2010
Guo Q, Ma J, Yuan B, Zhou M, Wu (2015) High-efficiency Agrobacterium-mediated transformation of Lotus corniculatus L. using phosphomannose isomerase positive selection. Plant Cell Tissue Organ Cult 121:413–422. doi:10.1007/s11240-015-0712-8
Hartmann HT, Kester DE, Davies FT, Geneve RL (1997) Plant propagation: principle and practice, 6th edn. Prentice-hall Inc, Upper Saddle River, pp 549–563
Heckmann AB, Sandal N, Bek AS, Madsen LH, Jurkiewicz A, Nielsen MW, Tirichine L, and Stougaard J (2011) Cytokinin induction of root nodule primordia in Lotus japonicus is regulated by a mechanism operating in the root cortex. Mol Plant Microbe Interact 24(11):1385–1395. The American Phytopathological Society. doi:10.1094/MPMI-05-11-0142
Hoque MI, Zahan MM, Sarker RH (2007) In vitro plant regeneration in mungbean (Vigna radiate (L.) Wilczek). Plant Tissue Cult Biotechnol 17(2):209–216
Ikeuchi M, Sugimoto K, Iwase A (2013) Plant callus: mechanisms of induction and repression. Plant Cell 25:3159–3173
Jana S, Sivanesan I, Jeong BR (2013) Effect of cytokinins on in vitro multiplication of Sophora tonkinensis. Asian Pac J Trop Biomed 3(7):549–553. doi:10.1016/S2221-1691(13)60111-2
Jeong BR, Sivanesan I (2016) Micropropagation, berberine content and antitumor activity of Jeffersonia dubia (Maxim.) Benth et Hook. Plant Cell Tissue Organ Cult 124:453–458. doi:10.1007/s11240-015-0898-9
Koné M, Patat-Ochatt EM, Conreux C, Sangwan RS, Ochatt SJ (2007) In vitro morphogenesis from cotyledon and epicotyl explants and flow cytometry distinction between landraces of Bambara groundnut [Vigna subterranea (L.) Verdc], an under-utilised grain legume. Plant Cell Tissue Organ Cult 88:61–75. doi:10.1007/s11240-006-9179-y
Koné T, Kouakou HT, Konaté S, Ochatt JS (2013) Plant regeneration via direct shoot organogenesis from cotyledon explants of Bambara groundnut, Vigna subterranea (L.) Verdc. Biotechnol Agron Soc Environ 17(4):584–592
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with Tobacco tissue cultures. Physio Plant 15:473–497
National Research Council (NRC) (2006) Lost crops of Africa: vegetables and yam bean development, security and cooperation policy and global affairs (DSC), vol II. The National Academies Press, Washington, pp 322–344
Oagile O (2005) African yam bean; morphology, clonal propagation and nitrogen fixation. PhD Thesis, School of Biosciences, University of Nottingham
Obembe OO, Khan T, Popoola JO (2011) High frequency multiple shoots induction and plant regeneration in six elite India cotton cultivars. Can J Pure Appl Sci 5(1):1385–1389
Ochatt SJ, Ponte ´caille C, Rancillac M (2000) The growth regulators used for bud regeneration and shoot rooting affect the competence for flowering and seed set in regenerated plants of protein peas. In vitro Cell Dev Biol Plants 36:188–193. doi:10.1007/s11627-000-0035-1
Ochatt SJ, Abirached-Darmency M, Marget P, Aubert G (2007) The Lathyrus paradox “poor men’s diet” or a remarkable genetic resource for protein legume breeding? In: Ochatt SJ, Jain SM (eds) Breeding of neglected and under-utilised crops, spices and herbs. Science Press, Plymouth, pp 41–60
Oganale D (2009). Nodulation and nitrogen fixation of African yam beam (Sphenostylis stenocarpa). In: Proceedings of African crop science society conference, Cape Town. Science and technology supporting food security in Africa. September 28–October 1 2009
Ogunsola KE, Ilori CO (2008) In vitro propagation of miracle berry (Synsepalum dulcificum Daniel) through embryo and nodal cultures. Afr J Biotechnol 7(3):244–248
Onyenekwe PC, Njoku GC, Ameh DA (2000) Effect of cowpea processing methods on flatus causing oligosaccharides. Nutr Res 20:349–358
Piwowarczyk B, Pindel A (2014) Early stages of somatic embryogenesis in root callus of grass pea (Lathyrus sativus L.). J Cent Eur Agric 15(3):209–218
Piwowarczyk B, Tokarz K, Kamińska I (2016) Responses of grass pea seedlings to salinity stress in in vitro culture conditions. Plant Cell Tissue Organ Cult 124:227–240. doi:10.1007/s11240-015-0887-z
Polisetty R, Paul V, Deveshawr JJ, Kijetarpal S, Suresh K, Chandra R (1997) Multiple shoot induction by benzyladenine and complete plant regeneration from seed explants of chickpea (Cicer arietinum L.). Plant Cell Rep 16:565–571
Popoola JO, Adegbite AE, Obembe OO (2011) Cytological studies on some accessions of African yam bean (AYB) (Sphenostylis stenocarpa Hochst. Ex. A. Rich. Harms). Int Res J Plant Sci 2(8):249–253
Rout GR, Samantaray S, Das P (2000) In vitro manipulation and propagation of medicinal plants. Biotechnol Adv 18:91–120
Saka JO, Ajibade SR, Adeniyan ON, Olowoyo RB, Ogunbodede BA (2004) Survey of under-utilized grain legume production systems in south-west agricultural zone of Nigeria. J Agric Food Inf 6(2/3):93–108
SAS (Statistical Analysis System) (2010) Users guide. Basic version, 9.3. SAS Institute, Cary
Sasaki T, Suzaki T, Soyano T, Kojima M, Sakakibara H, Masayoshi Kawaguchi M (2014) Shoot-derived cytokinins systemically regulate root nodulation. Nat Commun 5:4983. doi:10.1038/ncomms5983
Singh ND, Sahoo L, Sarin NB, Jaiwal PK (2003) The effect of TDZ on organogenesis and somatic embryogenesis in pigeon pea (Cajanus cajan L. Millsp). Plant Sci 164:341–347
Singh R, Yadav R, Amla DV, Sanyal I (2016) Characterization and functional validation of two scaffold attachment regions (SARs) from Cicer arietinum (L.). Plant Cell Tissue Organ Cult 125:135–148. doi:10.1007/s11240-015-0935-8
Taji A, Kumar PP, Lakshmanan P (2002) In vitro plant breeding. Food Products Press, New York, p 167
Tripathi L, Singh AK, Singh S, Singh R, Chaudhary S, Sanyal I, Amla DV (2013) Optimization of regeneration and Agrobacterium-mediated transformation of immature cotyledons of chickpea (Cicer arietinum L.). Plant Cell Tissue Organ Cult 113:513–527. doi:10.1007/s11240-013-0293-3
Tubic L, Savic J, Mitic N, Milojevic J, Janosevic D, Budimir S, Zdravkovic-Korac S (2016) Cytokinins differentially affect regeneration, plant growth and antioxidative enzymes activity in chive (Allium schoenoprasum L.). Plant Cell Tissue Organ Cult. doi:10.1007/s11240-015-0869-1
Uguru MI, Madukaife SO (2001) Studies on the variability in agronomic and nutritive characteristics of African yam bean (Sphenostylis stenocarpa Hochst ex. A. Rich. Harms). Plant Prod Res J 6:10–19
Varshney RK, Close TJ, Singh NK, Hoisington DA, Cook DR (2009) Orphan legume crops enter the genomics era! Curr Opin Plant Biol 12:202–210. doi:10.1016/j.pbi.2008.12.004
Yadav PBS, Padmaja V (2003) Shoot organogenesis and plantlet regeneration from leaf of pigeon pea. Plant Cell Tissue Organ Cult 73:197–200
Yadav SK, Sreenu P, Vanaja M, Venkateswarlu B (2010) Efficient shoo Regeneration from double cotyledonary node explants of green gram Vigna radiata (L.) Wilczek. Indian J Biotechnol 9:403–407
Acknowledgments
We thank the management of the National Centre for Genetic Resources and Biotechnology (NACGRAB), Moor Plantation, Ibadan, Nigeria, for making their in vitro Culture Laboratory facilities available for this study.
Authors contribution
Ogunsola K.E., Designed the experiment, monitored and coordinated the research activities, carried out parts of the data analysis and wrote the manuscript; Ojuederie O.B., Sourced the African yam bean seeds, performed parts of the in vitro culture activities, monitored data collection, carried out parts of the data analysis and reviewed the manuscript; Emmanuel B., Performed parts of the in vitro culture activities, carried out laboratory maintenance and other laboratory technologist functions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogunsola, K.E., Ojuederie, O.B. & Emmanuel, B. In vitro morphogenic responses of African yam bean (Sphenostylis stenocarpa (Hochst ex. A. Rich.) Harms) accessions to plant growth regulators. Plant Cell Tiss Organ Cult 127, 613–622 (2016). https://doi.org/10.1007/s11240-016-1036-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1036-z