Abstract
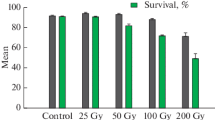
Artemisia annua L. is a commercial source of artemisinin. Nevertheless, artemisinin content within the plant is relatively low and varies depending on genotype and environment. To broaden the genetic variability, the mutation effect of 12C-ion beam irradiation on A. annua was examined. Irradiation at 2.5 Gy had a slight lethal effect to nodal segments while a noticeable lethal effect was observed at 5 and 10 Gy. Furthermore, at higher doses (20 and 50 Gy), a severe lethal effect was observed. Mutations at the DNA level of axillary bud-derived shoots were performed by RAPD. The mutation frequency at 10 Gy was about 1.7 and 2.1 times higher than that at 2.5 and 5 Gy, respectively. After growth and artemisinin production observation of 72 irradiated mutants, around 14 and 7 % of them showed higher artemisinin content and artemisinin yield compared to the controls, respectively. The highest artemisinin content in a mutant was 1.43 % DW, which was 3.2-fold higher than the original wild type. Additionally, the highest artemisinin yield in mutants was 3.68 mg/plant, which was around 1.4-fold higher than in the wild type. Moreover, irradiated mutants exhibited antibacterial activity against S. aureus, but the wild types did not. This study presents an effective application of heavy ion beam irradiation to create variations and improve artemisinin production in A. annua.




Similar content being viewed by others
References
Amano J, Kuwayama S, Mizuta Y, Oomiya T, Nakamura T, Nakano M (2007) Early identification of intra- and intergeneric hybrids among Colchicaceous ornamentals, Gloriosa spp., Littonia modesta Hook. and Sandersonia aurantiaca Hook., by flow cytometry and random amplified polymorphic DNA analysis. J Japan Soc Hort Sci 76:73–78
Banyai W, Mii M, Supaibulwatana K (2011) Enhancement of artemisinin content and biomass in Artemisia annua by exogenous GA3 treatment. Plant Growth Regul 63:45–54
Baraldi R, Isacchi B, Predieri S, Marconi G, Vincieri FF, Bilia AR (2008) Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem Syst Ecol 36:340–348
Chen YF, Chen W, Huang X, Hu X, Zhao JT, Gong Q, Li XJ, Huang XL (2013) Fusarium wilt-resistant lines of Brazil banana (Musa spp., AAA) obtained by EMS-induced mutation in a micro-cross-section cultural system. Plant Pathol 62:112–119
Datta SK, Misra P, Mandal AKA (2005) In vitro mutagenesis—a quick method for establishment of solid mutant in chrysanthemum. Curr Sci 88:155–158
Delabays N, Simonnet X, Gaudin M (2001) The genetics of artemisinin content in Artemisia annua L. and the breeding of high yielding cultivars. Current Med Chem 8:1795–1801
Dong X, Li W (2012) Biological features of an early-maturity mutant of sweet sorghum induced by carbon ions irradiation and its genetic polymorphism. Adv Space Res 50:496–501
Ferreira JFS, Janick J (1996) Distribution of artemisinin in Artemisia annua. In: Janick J (ed) Progress in new crops. ASHS Press, Arlington, pp 579–584
Ferreira JFS, Laughlin JC, Delabays N, de Magalhães PM (2005) Cultivation and genetics of Artemisia annua L for increased production of the antimalarial artemisinin. Plant Genet Resour 3:206–229
Ishikawa S, Ishimaru Y, Igura M, Kuramata M, Abe T, Senoura T, Hase Y, Arao T, Nishizawa NK, Nakanishi H (2012) Ion-beam irradiation, gene identification, and maker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci USA 109:19166–19171
Kanai T, Kohno T, Minohara S, Sudou M, Takada E, Soga F, Kawachi K, Fukumura A (1993) Dosimetry and measured differential W values of air for heavy ions. Radiat Res 135:293–301
Kazama Y, Hirano T, Saito H, Liu Y, Ohbu S, Hayashi Y, Abe T (2011) Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. BMC Plant Biol 11:161. doi:10.1186/1471-2229-11-161
Kazama Y, Fujiwara MT, Takehisa H, Ohbu S, Saito H, Ichida H, Hayashi Y, Abe T (2013) Characterization of a heavy-ion induced white flower mutant of allotetraploid Nicotiana tabacum. Plant Cell Rep 32:11–19
Kikuchi S, Saito Y, Ryto H, Fukunishi N, Abe T, Tanaka H, Tsujimoto H (2009) Effect of heavy ion beams on chromosomes of common wheat, Triticum aestivum. Mutat Res 669:63–66
Koobkokkruad T, Chochai A, Kirdmanee C, De-Eknamkul W (2008) Effects of low-dose gamma irradiation on artemisinin content and amorpha-4,11-diene synthase activity in Artemisia annua L. Int J Radiat Biol 84:878–884
Kumar S, Gupta SK, Singh P, Bajpai P, Gupta MM, Singh D, Gupta AK, Ram G, Shasany AK, Sharma S (2004) High yields of artemisinin by multi-harvest of Artemisia annua crops. Ind Crop Prod 19:77–90
Lu G, Zhang X, Zou Y, Zou Q, Xiang X, Cao J (2007) Effect of radiation on regeneration of Chinese narcissus and analysis of genetic variation with AFLP and RAPD markers. Plant Cell, Tissue Organ Cult 88:319–327
Magori S, Tanaka A, Kawaguchi M (2010) Physically induced mutation: ion beam mutagenesis. In: Meksem K, Kahl K (eds) The Handbook of plant mutation screening: mining of natural and induced. WILEY-VCH Verlag GmbH & Co KGaA, Weinheim, pp 3–16
Mandal AKA, Chakrabarty D, Datta SK (2000) Application of in vitro techniques in mutation breeding of chrysanthemum. Plant Cell, Tissue Organ Cult 60:33–38
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-essays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano M, Amano J, Watanabe Y, Nomizu T, Suzuki M, Mizunashi K, Mori S, Kuwayama S, Han D, Saito H, Ryuto H, Fukunishi N, Abe T (2010) Morphological variation in Tricyrtishirta plants regenerated from heavy ion beam-irradiated embryogenic calluses. Plant Biotechnol 27:155–160
Novak FJ, Brunner H (1992) Plant breeding: induced mutation technology for crop improvement. IAEA Bull 4:25–33
Okamura M, Yasuno N, Ohtsuka M, Tanaka A, Shikazono N, Hase Y (2003) Wide variety of flower-color and -shape mutants regenerated from leaf cultures irradiated with ion beams. Nucl Instr Methods Phys Res B 206:574–578
Othman M, Loh HS, Wiart C, Khoo TJ, Lim KH, Ting KN (2011) Optimal methods for evaluating antimicrobial activities from plant extracts. J Microbiol Methods 84:161–166
Roux NS, Dolezel J, Swennen R, Zapata-Arias FJ (2001) Effectiveness of three micropropagation techniques to dissociate cytochimeras in Musa spp. Plant Cell, Tissue Organ Cult 66:189–197
Sala CA, Bulos M, Echarte M, Whitt SR, Ascenzi R (2008) Molecular and biochemical characterization of an induced mutation conferring imidazolinone resistance in sunflower. Theor Appl Genet 118:105–112
Sangsiri C, Sorajjapinun W, Srinives P (2005) Gamma radiation induced mutation in mungbean. Scienceasia 31:251–255
Shi X, Li K, Nie Y, Zhang G (2006) Biochemistry and genetic analysis of bioeffects of low energy N+ Implantation and Y-radiation on Arabidopsis thaliana. Front Biol China 1:41–45
Shikazono N, Tanaka A, Kitayama S, Watanabe H, Tano S (2002) LET dependence of lethality in Arabidopsis thaliana irradiated by heavy ions. Radiat Environ Biophys 41:159–162
Shikazono N, Yokota Y, Kitamura S, Suzuki C, Watanabe H, Tano S, Tanaka A (2003) Mutation rate and novel tt mutants of Arabidopsis thaliana induced by carbon ions. Genetics 163:1449–1455
Shikazono N, Suzuki C, Kitamura C, Watanabe H, Tano S, Tanaka A (2005) Analysis of mutations induced by carbon ions in Arabidopsis thaliana. J Exp Bot 56:587–596
Shirao T, Ueno K, Abe T, Matsuyama T (2013) Development of DNA markers for identifying chrysanthemum cultivars generated by ion-beam irradiation. Mol Breed 31:729–735
Takahashi M, Kohama S, Kondo K, Hakata M, Hase Y, Shikazono N, Tanaka A, Morikawa H (2005) Effects of ion beam irradiation on the regeneration and morphology of Ficus thunbergii Maxim. Plant Biotechnol 22:63–67
Takahashi M, Kohama S, Shigeto J, Hase Y, Tanaka A, Morikawa H (2012) Mutants of Ficuspumila produced by ion beam irradiation with an improved ability to uptake and assimilate atmospheric nitrogen dioxide. Int J Phytoremediat 14:275–281
Takano N, Yamamoto M, Takahash Y, Teranishi M, Yamaguchi H, Sakamoto AN, Hase Y, Fujisawa H, Wu J, Matsumoto T, Toki S, Hidema J (2013) Isolation of a novel UVB-tolerant rice mutant obtained by exposure to carbon-ion beams. J Radiat Res 54:1–12
Tanaka A, Shikazono N, Yokota Y, Watanabe H, Tano S (1997) Effects of heavy ions on the germination and survival of Arabiopsis thaliana. Int J Radiat Biol 72:121–127
Tanaka A, Shikazono N, Hase Y (2010) Studies on biological effects of ion beams on lethality, molecular nature of mutation, mutation rate, and spectrum of mutation phenotype for mutation breeding in higher plants. J Radiat Res 51:223–233
Verma RK, Chauhan A, Verma RS, Gupta AK (2011) Influence of planting date on growth, artemisinin yield, seed and oil yield of Artemisia annua L. under sub temperate climatic conditions. Ind Crop Prod 34:860–864
WHO (2001) Antimalarial drug combination therapy: report of a technical consultation. World Health Organization, Geneva
WHO (2012) World malaria report 2012. World Health Organization, Geneva
Wu L, Yu Z (2001) Radiobiological effects of a low-energy ion beam on wheat. Radiat Environ Biophys 40:53–57
Wu LD, Hou SW, Qian PP, Sun LD, Zhang YC, Li WJ (2009) Flower color chimera and abnormal leaf mutants induced by 12C6+ heavy ions in Salvia splendens Ker-Gawl. Sci Hort 121:462–467
Yamaguchi H, Shimizu A, Hase Y, Degi K, Tanaka A, Morishita T (2009) Mutation induction with ion beam irradiation of lateral buds of chrysanthemum and analysis of chimeric structure of induced mutants. Euphytica 165:97–103
Yang H, Schmidt H (1994) Selection of a mutant from adventitious shoots formed in X ray treated cherry leaves and differentiation of standard and mutant with RAPDs. Euphytica 77:89–92
Yokota Y, Yamada S, Hase Y, Shikazono N, Narumi I, Tanaka A, Inoue M (2007) Initial yields of DNA double-strand breaks and DNA fragmentation patterns depend on linear energy transfer in tobacco BY-2 protoplasts irradiated with helium, carbon and neon ions. Radiat Res 167:94–101
Zhang L, Ye HC, Li GF (2006) Effect of development stage on the artemisinin content and the sequence characterized amplified region (SCAR) marker of high-artemisinin yielding strains of Artemisia annua L. J Integr Plant Biol 48:1054–1062
Zhou LB, Li WJ, Yu LX, Li P, Li Q, Ma S, Dong XC, Zhou GM, Leloup C (2006) Linear energy transfer dependence of the effects of carbon ion beams on adventitious shoot regeneration from in vitro leaf explants of Saintpaulia ionahta. Int J Radiat Biol 82:473–481
Acknowledgments
The authors would like to thank the Office of the Higher Education Commission, Thailand for their support by providing support under the Strategic Scholarships for Frontier Research Network program for the Joint Ph.D. Program Thai Doctoral degree. We owe our sincere gratitude to the Graduate School of Science and Technology, Niigata University, Japan for financial assistance under the Global Circus Project during research at Niigata University, Japan. Thanks also due to Faculty of Science, Mahidol University, Thailand for facilities and research grant.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Inthima, P., Otani, M., Hirano, T. et al. Mutagenic effects of heavy-ion beam irradiation on in vitro nodal segments of Artemisia annua L.. Plant Cell Tiss Organ Cult 119, 131–139 (2014). https://doi.org/10.1007/s11240-014-0519-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0519-z