Abstract
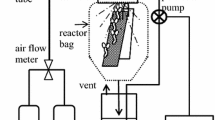
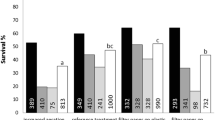
A mist bioreactor using a disposable bag as culture chamber was used to propagate carrot embryogenic cells into rooted plantlets. The best operating configuration was akin to a vertical hanging garden using 50–90 μm nylon mesh for explant attachment. Cells spray inoculated into the reactor were 51.2 % viable. Misting cycle and aeration conditions were studied and showed that under the same hourly volumetric nutrient feed and 0 VVM, embryo development in the reactor was best using a 0.3 min on/2.7 min off misting cycle, yielding about 23 % post heart stage embryos. Compared to 0 VVM, 3 % CO2 enrichment improved embryo development in reactor culture. Spray inoculated cells also attached to several vertically hung poly-l-lysine coated strips and then developed in situ into embryos. Cell attachment was significantly improved when they were suspended in salt-free sucrose solution during spray inoculation. Almost 90 % of the originally attached cells remained on the nylon mesh 24 h later after spraying with B5 medium in the mist reactor. Strip grown embryos had the same post heart stage ratio but shorter overall length compared to those developed on a horizontal platform. Young plantlets developed uniformly up and down the hanging strips and did not detach after 3 weeks of culture suggesting this technology may prove useful for improving micropropagation.






Similar content being viewed by others
Abbreviations
- N50, N70, N90:
-
Nylon screen/mesh with openings of 50, 70 and 90 μm, respectively
- P74, P105:
-
Polypropylene screen/mesh with opening of 74 and 105 μm, respectively
- PLL:
-
Poly-l-lysine
References
Adelberg J, Fári MG (2010) Applied physiology and practical bioreactors for plant propagation. Propag Ornam Plants 10:205–219
Afreen F (2006) Temporary immersion bioreactor-engineering considerations and applications in plant micropropagation. In: Dutta Gupta S, Ibaraki Y (eds) Plant tissue culture engineering. Focus on biotechnology, vol 6. Springer, Heidelberg, pp 187–201
Afreen F, Zobayed SMA, KozaiI T (2002) Photoautotrophic culture of Coffea arabusta somatic embryos: photosynthetic ability and growth of different stage embryos. Ann Bot 90:11–19. doi:10.1093/aob/mcf150
Aitken-Christie J, Kozai T, Takayama S (1995) Automation in plant tissue culture: general introduction and overview. In: Kozai T, Smith MAL, Aitken-Christie J (eds) Automation and environmental control in plant tissue culture. Kluwer, Boston, pp 1–18
Albarrán J, Bertrand B, Lartaud M, Etienne H (2005) Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water and mineral status of coffee (Coffea arabica) somatic embryos. Plant Cell Tiss Organ Cult 81:27–36. doi:10.1007/s11240-004-2618-8
Barbón R, Jiménez E, Preil W (2008) Influence of in vitro environment on somatic embryogenesis of Coffea arabica L. cv. Caturra rojo: the effects of carbon dioxide on embryogenic cell suspensions. Plant Cell Tiss Organ Cult 95:155–161. doi:10.1007/s11240-008-9427-4
Buddendorfjoosten JMC, Woltering EJ (1994) Components of the gaseous environment and their effects on plant growth and development in vitro. Plant Growth Regul 15:1–16
Chung C-M, Bae KKA, Masatoshi (2000) Effects of CO2 enrichment on the differenciation and growth in tissue culture of Panax ginseng C. A. Meyer. Korean J Medicinal Crop Sci 8:14–20
Correll MJ, Weathers PJ (2001a) Effects of light, CO2 and humidity on carnation growth, hyperhydration and cuticular wax development in a mist reactor. In Vitro Cell Dev Biol Plant 37:405–413. doi:10.1007/s11627-001-0071-5
Correll MJ, Weathers PJ (2001b) One-step acclimatization of plantlets using a mist reactor. Biotechnol Bioeng 73:253–258. doi:10.1002/bit.1058
Correll MJ, Wu Y, Weathers PJ (2001) Controlling hyperhydration of carnations (Dianthus caryophyllus L.) grown in a mist reactor. Biotechnol Bioeng 71:307–314. doi:10.1002/1097-0290(2000)71:4<307:AID-BIT1019>3.0.CO;2-9
DiIorio A, Cheetham R, Weathers P (1992) Carbon dioxide improves the growth of hairy roots cultured on solid medium and in nutrient mists. Appl Microbiol Biot 37:463–467. doi:10.1007/bf00180969
Ducos J-P, Terrier B, Courtois D (2009) Disposable bioreactors for plant micropropagation and mass plant cell culture. Adv Biochem Eng/Biotechnol 115:89–115. doi:10.1007/10_2008_28
Etienne H, Berthouly M (2002) Temporary immersion systems in plant micropropagation. Plant Cell Tiss Organ Cult 69:215–231. doi:10.1023/a:1015668610465
Facchini PJ, Wilhelm Neumann A, DiCosmo F (1989) Adhesion of suspension-cultured Catharanthus roseus cells to surfaces: effect of pH, ionic strength, and cation valency. Biomaterials 10:318–324. doi:10.1016/0142-9612(89)90072-0
Fari MG, Kertesz T, Laszlo M, Varga Z (2006) Design of a revert rotary plant micropropagation bioreactor-’3R’ system: application for research, possibilities of scaling-up and limitations. Acta Hortic 725:561–570
Fisichella M, Morini S (2003) Somatic embryo and root regeneration from quince leaves cultured in ventilated vessels or under different oxygen and carbon dioxide levels. In Vitro Cell Dev Biol Plant 39:402–408. doi:10.1079/ivp2003429
Gamborg O, Murashige T, Thorpe T, Vasil I (1976) Plant tissue culture media. In Vitro Cell Dev Biol Plant 12:473–478. doi:10.1007/bf02796489
Grafen A, Hails R (2002) Modern statistics for the life sciences. Oxford University Press, Oxford, pp 241–243
Guo Z, Zeng Z, Liu R, Deng Y (2005) Morphological transformation of plant cells in vitro and its effect on plant growth. Tsinghua Sci Technol 10:573–578. doi:10.1016/s1007-0214(05)70120-6
Hazarika BN (2006) Morpho-physiological disorders in in vitro culture of plants. Sci Hortic 108:105–120. doi:10.1016/j.scienta.2006.01.038
Huang SY, Chan HS, Wang TT (2006) Induction of somatic embryos of celery by control of gaseous compositions and other physical conditions. Plant Growth Regul 49:219–227. doi:10.1007/s10725-006-9113-7
Jay V, Genestier S, Courduroux J-C (1992) Bioreactor studies on the effect of dissolved oxygen concentrations on growth and differentiation of carrot (Daucus carota L.) cell cultures. Plant Cell Rep 11:605–608. doi:10.1007/bf00236382
Jeong C-S, Chakrabarty D, Hahn E-J, Lee H-L, Paek K-Y (2006) Effects of oxygen, carbon dioxide and ethylene on growth and bioactive compound production in bioreactor culture of ginseng adventitious roots. Biochem Eng J 27:252–263. doi:10.1016/j.bej.2005.08.025
Li H, Kurata K (2005) Static suspension culture of carrot somatic embryos. J Biosci Bioeng 99:300–302. doi:10.1263/jbb.99.300
Liu CZ, Towler MJ, Medrano G, Cramer CL, Weathers PJ (2009) Production of mouse interleukin-12 is greater in tobacco hairy roots grown in a mist reactor than in an airlift reactor. Biotechnol Bioeng 102:1074–1086. doi:10.1002/bit.22154
Loberant B, Altman A (2010) Micropropagation of plants. In: Flickinger MC (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. Wiley, New York, pp 3499–3515
Lowe KC, Anthony P, Power JB, Davey MR (2003) Invited review: novel approaches for regulating gas supply to plant systems in vitro: Application and benefits of artificial gas carriers. In Vitro Cell Dev Biol: Plant 39:557–566. doi:10.1079/ivp2003469
Paek KY, Hahn EJ, Son SH (2001) Application of bioreactors for large-scale micropropagation systems of plants. In Vitro Cell Dev Biol: Plant 37:149–157. doi:10.1007/s11627-001-0027-9
Paek KY, Chakrabarty D, Hahn EJ (2005) Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tiss Organ Cult 81:287–300. doi:10.1007/s11240-004-6648-z
Pospisilova J, Ticha I, Kadlecek P, Haisel D, Plzakova S (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497. doi:10.1023/A:1002688208758
Robert ML, Herrera-Herrera JL, Herrera-Herrera G, Herrera-Alamillo MÁ, Fuentes-Carrillo P (2006) A new temporary immersion bioreactor system for micropropagation. Methods Mol Biol 318:121–129. doi:10.1385/1-59259-959-1:121
Rosnow J, Offermann S, Park J, Okita T, Tarlyn N, Dhingra A, Edwards G (2011) In vitro cultures and regeneration of Bienertia sinuspersici (Chenopodiaceae) under increasing concentrations of sodium chloride and carbon dioxide. Plant Cell Rep 30:1541–1553. doi:10.1007/s00299-011-1067-1
Roustan JP, Latche A, Fallot J (1989) Stimulation of Daucus carota somatic embryogenesis by inhibitors of ethylene synthesis: cobalt and nickel. Plant Cell Rep 8:182–185. doi:10.1007/bf00716836
Roustan JP, Latche A, Fallot J (1990) Inhibition of ethylene production and stimulation of carrot somatic embryogenesis by salicylic acid. Biol Plant 32:273–276. doi:10.1007/bf02886947
Roustan J-P, Latché A, Fallot J (1994) Role of ethylene on induction and expression of carrot somatic embryogenesis: relationship with polyamine metabolism. Plant Sci 103:223–229. doi:10.1016/0168-9452(94)90210-0
Shaik S, Dewir YH, Singh N, Nicholas A (2010) Micropropagation and bioreactor studies of the medicinally important plant Lessertia (Sutherlandia) frutescens L. S Afr J Bot 76:180–186. doi:10.1016/j.sajb.2009.10.005
Shigeta J, Sato KJ, Mii M (1996) Effects of initial cell density, pH and dissolved oxygen on bioreactor production of carrot somatic embryos. Plant Sci 115:109–114. doi:10.1016/s0168-9452(96)04327-o
Shimazu T, Kurata K (1999) Relationship between production of carrot somatic embryos and dissolved oxygen concentration in liquid culture. Plant Cell Tiss Organ Cult 57:29–38. doi:10.1023/a:1006267002706
Shoji M, Sato H, Nakagawa R, Funada R, Kubo T, Ogita S (2006) Influence of osmotic pressure on somatic embryo maturation in Pinus densiflora. J For Res 11:449–453. doi:10.1007/s10310-006-0227-6
Shomer I, Novacky AJ, Pike SM, Yermiyahu U, Kinraide TB (2003) Electrical potentials of plant cell walls in response to the ionic environment. Plant Physiol 133:411–422. doi:10.1104/pp.103.024539
Snyman SJ, Nkwanyana PD, Watt MP (2011) Alleviation of hyperhydricity of sugarcane plantlets produced in RITA (R) vessels and genotypic and phenotypic characterization of acclimated plants. S Afr J Bot 77:685–692. doi:10.1016/j.sajb.2011.03.004
Takamura T, Imose Y, Tanaka M (2010) Effect of CO2 enrichment on in vitro plant regeneration through somatic embryogenesis in cyclamen (Cyclamen persicum mill.). Kagawa Daigaku Nogakubu Gakujutsu Hokoku 62:1–4
Tate J, Payne G (1991) Plant cell growth under different levels of oxygen and carbon dioxide. Plant Cell Rep 10:22–25. doi:10.1007/bf00233026
Towler MJ, Weathers PJ (2003) Adhesion of plant roots to poly-l-lysine coated polypropylene substrates. J Biotechnol 101:147–155. doi:10.1016/S0168-1656(02)00319-X
Towler MJ, Kim Y, Wyslouzil BE, Correll M, Weathers PJ (2006) Design, development, and applications of mist bioreactors for micropropagation and hairy root culture. In: Ibaraki Y, Gupta SD (eds) Plant tissue culture engineering. Focus on biotechnology, vol 6. Springer, Heidelberg, pp 119–134
Wang YS, Tong Y, Li YF, Zhang Y, Zhang J, Feng JY, Feng H (2011) High frequency plant regeneration from microspore-derived embryos of ornamental kale (Brassica oleracea L. var. acephala). Sci Hortic 130:296–302. doi:10.1016/j.scienta.2011.06.029
Weathers PJ, Zobel RW (1992) Aeroponics for the culture of organisms, tissues and cells. Biotechnol Adv 10:93–115. doi:10.1016/0734-9750(92)91353-G
Weathers PJ, Towler MJ, Xu JF (2010) Bench to batch: advances in plant cell culture for producing useful products. Appl Microbiol Biot 85:1339–1351. doi:10.1007/s00253-009-2354-4
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Wyslouzil BE, Waterbury RG, Weathers PJ (2000) The growth of single roots of Artemisia annua in nutrient mist reactors. Biotechnol Bioeng 70:143–150. doi:10.1002/1097-0290(20001020)70:2<143:aid-bit3>3.0.co;2-b
Ziv M (1991) Morphogenic patterns of plants micropropagated in liquid medium in shaken flasks or large scale bioreactor cultures. Israel J Bot 40:145–153
Ziv M (2010) Bioreactor technology for plant micropropagation. In: Janick J (ed) Horticultural reviews. Wiley, New York, pp 1–30
Acknowledgments
The authors thank WPI for supporting L Fei. Advice from Dr. Melissa Towler and Prof. Elizabeth Ryder of the Department of Biology and Biotechnology at WPI was greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fei, L., Weathers, P.J. From cells to embryos to rooted plantlets in a mist bioreactor. Plant Cell Tiss Organ Cult 116, 37–46 (2014). https://doi.org/10.1007/s11240-013-0380-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0380-5