Abstract
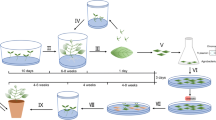
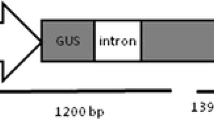
A protocol for adventitious shoot formation in Symphyotrichum novi-belgii was developed after investigating the effects of cultivar and hormone combinations. A Murashige and Skoog medium with 1.0 mg l−1 6-benzyladenine induced adventitious shoot formation in 15 out of 19 cultivars. Addition of 0.1 mg l−1 indole-3-acetic acid or naphthaleneacetic acid increased the total number of shoots per explant, but not the number of shoots longer than 1 cm. Addition of dichlorophenoxyacetic acid (2,4-D) promoted callus formation, but inhibited shoot elongation. A transformation system for the two cultivars Victoria Fanny and Victoria Jane was developed by co-cultivation of leaf explants with Agrobacterium tumefaciens. Three bacterial strains (LBA 4404, A281 and C58) all carrying the binary vector, p35S-GUS-INT, and harbouring the uidA gene coding for β-glucuronidase (GUS) were used. Regeneration of transgenic plants after co-cultivation with A281 was independent of cultivar, and all explants produced callus followed by indirect shoot formation. In ‘Victoria Fanny’ shoots were formed faster and without a callus phase after co-cultivation with LBA 4404 or C58. The highest number of potentially transformed shoots was regenerated after co-cultivation of ‘Victoria Fanny’ leaf explants with LBA 4404. Integration of the transgenes in the plant genome was confirmed using PCR and Southern blot hybridisation. To verify that the transgenes could be transferred to offspring, crosses were conducted between three transgenic lines of ‘Victoria Fanny’ and two wild type pollen donors. It was demonstrated that viable seeds were produced and that the uidA gene was inherited.






Similar content being viewed by others
References
Anon (2011) Oversigt over de mængdemæssigt største kulturer produceret i Danmark 2004–2009. Danske prydplanter. http://www.floradania.dk/fileadmin/templates/PDF-filer/Branchenyt/Top_14/Oversigt_over_alle _lister_2004-2009.pdf. Accessed 2 Sep 2011
Bennici A, Anzidei M, Vendramin GG (2004) Genetic stability and uniformity of Foeniculum vulgare Mill. regenerated plants through organogenesis and somatic embryogenesis. Plant Sci 166:221–227
Cammareri M, Errico A, Filippone E, Conicella C (2001) Screening of aster wild germplasm for in vitro response to regeneration. J Gen Breed 55:255–260
Cammareri M, Errico A, Filippone E, Esposito S, Conicella C (2002) Induction of variability in chimeric Aster cordifolius ‘White Elegans’ through somaclonal variation. Euphytica 128:19–25
Clark M (1997) Plant molecular biology—a laboratory manual. Springer, New York
Corral P, Mallon R, Rodriguez-Oubina J, Gonzalez ML (2011) Multiple shoot induction and plant regeneration of the endangered species Crepis novoana. Plant Cell Tiss Organ Cult 105:211–217
Cui ML, Ezura H, Nishimura S, Kamada H, Handa T (2004) A rapid Agrobacterium-mediated transformation of Antirrhinum majus L. by using direct shoot regeneration from hypocotyl explants. Plant Sci 166:873–879
da Silva JAT (2004) Ornamental chrysanthemums: improvement by biotechnology. Plant Cell Tiss Organ Cult 79:1–18
Desgagnes R, Laberge S, Allard G, Khoudi H, Castonguay Y, Lapointe J, Michaud R, Vezina LP (1995) Genetic transformation of commercial breeding lines of alfalfa (Medicago sativa). Plant Cell Tiss Organ Cult 42:129–140
Dessaux Y, Petit A, Tempé J (1992) Opines in Agrobacterium biology. In: Verma DPS (ed) Molecular signals in plant-microbe communications. CRC Press, USA, pp 109–136
Dhar U, Joshi M (2005) Efficient plant regeneration protocol through callus for Saussurea obvallata (DC.) Edgew. (Asteraceae): effect of explant type, age and plant growth regulators. Plant Cell Rep 24:195–200
Gallagher SA (1992) GUS protocols: using the GUS gene as a reporter of gene expression. Academic Press, USA
Garfinkel DJ, Nester EW (1980) Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol 144:732–743
Guo HM, Zhang YQ, Wan FH, Cheng HM (2010) Agrobacterium-mediated transformation of Eupatorium adenophorum. Plant Cell Tiss Organ Cult 103:417–422
Gürel E, Kazan K (1999) Evaluation of various sunflower (Helianthus annuus L.) genotypes for Agrobacterium tumefaciens-mediated gene transfer. Turk J Bot 23:171–177
Hassanein A, Chevreau E, Dorion N (2005) Highly efficient transformation of zonal (Pelargonium × hortorum) and scented (P. capitatum) geraniums via Agrobacterium tumefaciens using leaf discs. Plant Sci 169:532–541
Hassanein A, Hamama L, Loridon K, Dorion N (2009) Direct gene transfer study and transgenic plant regeneration after electroporation into mesophyll protoplasts of Pelargonium × hortorum, ‘Panach, Sud’. Plant Cell Rep 28:1521–1530
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Karami O, Esna-Ashari M, Kurdistani GK, Aghavaisi B (2009) Agrobacterium mediated genetic transformation of plants: the role of host. Biol Plantarum 53:201–212
Kishimoto S, Aida R, Shibata M (2002) Agrobacterium tumefaciens mediated transformation of elatior begonia (Begonia × hiemalis fotsch). Plant Sci 162:697–703
Krishnamohan A, Balaji V, Veluthambi K (2001) Efficient vir gene induction in Agrobacterium tumefaciens requires virA, virG, and vir box from the same Ti plasmid. J Bacteriol 183:4079–4089
Kristiansen K, Petersen K (2009) In vitro mutagenesis in Aster novi-belgii cultivars. Acta Hort 836:207–214
Kristiansen K, Hansen CW, Brandt K (1997) Flower induction in seedlings of Aster novi-belgii and selection before and after vegetative propagation. Euphytica 93:361–367
Kunz C, Schob H, Leubner-Metzger G, Glazov E, Meins F (2001) Beta-1,3-glucanase and chitinase transgenes in hybrids show distinctive and independent patterns of posttranscriptional gene silencing. Planta 212:243–249
Lee M, Phillips RL (1988) The chromosomal basis of somaclonal variation. Annu Rev Plant Physiol Plant Mol Biol 39:413–437
Lee T, Huang MEE, Pua EC (1997) High frequency shoot regeneration from leaf disc explants of garland chrysanthemum (Chrysanthemum coronarium L.) in vitro. Plant Sci 126:219–226
Mercuri A, De Benedetti L, Burchi G, Schiva T (2000) Agrobacterium mediated transformation of African violet. Plant Cell Tiss Organ Cult 60:39–46
Mishiba KI, Ishikawa K, Tsujii O, Mii M (2000) Efficient transformation of lavender (Lavandula latifolia Medicus) mediated by Agrobacterium. J Hort Sci Biotechnol 75:287–292
Mørk EK, Kristiansen K, Jorgensen HJL, Sundelin T (2011) First report of Golovinomyces cichoracearum as the causal agent of powdery mildew on Symphyotrichum novi-belgii (synonym Aster novi-belgii) in Denmark. Plant Dis 95:228
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nadolska-Orczyk A, Orczyk W (2000) Study of the factors influencing Agrobacterium mediated transformation of pea (Pisum sativum L.). Mol Breed 6:185–194
Ntui VO, Khan RS, Chin DP, Nakamura I, Mii M (2010) An efficient Agrobacterium tumefaciens mediated genetic transformation of “Egusi” melon (Colocynthis citrullus L.). Plant Cell Tiss Organ Cult 103:15–22
Pniewski T, Kapusta J, Pucienniczak A (2006) Agrobacterium-mediated transformation of yellow lupin to generate callus tissue producing HBV surface antigen in a long-term culture. J Appl Genet 47:309–318
Pret’ova A, Obert B, Wetzstein HY (2001) Leaf developmental stage and tissue location affect the detection of beta-glucuronidase in transgenic tobacco plants. Biotechnol Lett 23:555–558
Salazar R, Vargas TE, De Garcia E, Oropeza M (2005) Micropropagation and organogenesis were established for Aster ericoides cv. “Monte Cassino”. Interciencia 30:295–299
Sambrook J, Fritsch EF, Marchive C (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York
Shilpa KS, Kumar VD, Sujatha M (2010) Agrobacterium-mediated genetic transformation of safflower (Carthamus tinctorius L.). Plant Cell Tiss Organ Cult 103:387–401
Sreedhar RV, Venkatachalam L, Thimmaraju R, Bhagyalakshmi N, Narayan MS, Ravishankar GA (2008) Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biol Plant 52:355–360
Sujatha M, Kumar VD (2007) In vitro bud regeneration of Carthamus tinctorius and wild Carthamus species from leaf explants and axillary buds. Biol Plant 51:782–786
Uno Y, Nakao S, Yamai Y, Koyama R, Kanechi M, Inagaki N (2009) Callus formation, plant regeneration, and transient expression in the halophyte sea aster (Aster tripolium L.). Plant Cell Tiss Organ Cult 98:303–309
Vanegas PE, Cruz-Hernandez A, Valverde ME, Paredes-Lopez O (2002) Plant regeneration via organogenesis in marigold. Plant Cell Tiss Organ Cult 69:279–283
Viterbo A, Rabinowitch HD, Altman A (1992) Plant regeneration from callus cultures of Allium trifoliatum subsp. hirsutum and assessment of genetic stability by isozyme polymorphism. Plant Breed 108:265–273
Wang T, Atkinson R, Janssen B (2007) The choice of Agrobacterium strain for transformation of kiwifruit. In: Ferguson AR, Hewett EW, Gunson FA, Hale CN (eds) Proceedings of the 6th international symposium on kiwifruit, pp 227–232
Wildi E, Schaffner W, Buter KB (1998) In vitro propagation of Petasites hybridus (Asteraceae) from leaf and petiole explants and from inflorescence buds. Plant Cell Rep 18:336–340
Zalewska M, Lema-Ruminska J, Miler N (2007) In vitro propagation using adventitious buds technique as a source of new variability in chrysanthemum. Sci Hort 113:70–73
Acknowledgments
The authors want to thank Mrs. Elisabeth Kjemtrup-Hansen for expert technical assistance. The research was funded by the Danish Aster Association and The Danish Food Industry Agency under the Ministry of Food, Agriculture and Fisheries (project No. 536-07-654).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mørk, E.K., Henriksen, K., Brinch-Pedersen, H. et al. An efficient protocol for regeneration and transformation of Symphyotrichum novi-belgii . Plant Cell Tiss Organ Cult 108, 501–512 (2012). https://doi.org/10.1007/s11240-011-0065-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-0065-x