Abstract
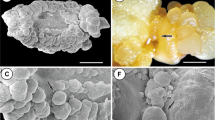
The ontogenetic developmental stages of saffron somatic embryogenesis have been studied and characterized using light microscopy and the biochemical determination of the antioxidant enzymatic system. The embryogenic callus underwent internal segmented divisions with the formation of globular embryos that were attached to the callus surface by a broad multicellular structure. Further development of the embryoids was characterized by the emergence of a shoot apical meristem and cotyledon (monopolar stage) with the subsequent differentiation of a minicorm at the basal part of the somatic embryo (dipolar stage). During the morphological differentiation of the somatic embryos changes in the antioxidant enzymatic system with increased superoxide dismutase (SOD) and catalase (CAT) activities were detected at the initial stages of somatic embryogenesis. The isoforms of SOD, including two Mn-SODs and four Cu, Zn-SODs, were also detected. Although all the isoforms were expressed during the successive stages of somatic embryogenesis, an increase in Mn-SODs and a decrease in Cu, Zn-SODs during the last two stages was observed. Significant changes were also detected in the antioxidant activities ascorbate peroxidase, dehydroascorbic acid reductase and glutathione reductase.







Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbate
- BA:
-
Benzyladenine
- CAT:
-
Catalase
- 2,4 D:
-
2,4 Dichlorophenoxiacetic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- DHA:
-
Dehydroascorbic acid
- DHAR:
-
Dehydroascorbic acid reductase
- GR:
-
Glutathione reductase
- GSSG:
-
Glutathione oxidized
- GSH:
-
Glutathione reduced
- KCN:
-
Potassium cyanide
- MDA:
-
Malondialdehyde
- MDHAR:
-
Monodehydroascorbate reductase
- NAA:
-
α-Naphtaleneacetic acid
- NADH:
-
Nicotine adenine dinucletide reduced
- MS:
-
Murashige and Skoog 1962
- PAGE:
-
Polyacryamide gel electrophoresis
- PMSF:
-
Phenylmethylsulphonilfluoride
- p-HMB:
-
p-Hydroxy mercury benzoic acid
- PVP:
-
Polyvinylpyrrolidone
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Adams L, Benson EE, Staines HJ, Bremmer DH, Millan S, Dighton N (1999) Effects of the lipid peroxidation products 4-hydroxy-2-nonenal and malondialdehyde on the proliferation and morphogenic development of in vitro plan cells. J Plant Physiol 155:376–386
Aebi M (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Ahuja A, Koul A, Ram G, Kaul BL (1994) Somatic embryogenesis and plant regeneration in saffron, Crocus sativus L. Indian J Exp Biol 32:135–140
Bagnoli F, Capuana M, Racchi NL (1998) Developmental changes in catalase and superoxide dismutase isoenzymes in zygotic and somatic embryos of horse chestnut. Funct Plant Biol 25:909–913. doi:10.1071/PP98068
Bartel B, Le Clere S, Magidin M, Zolman BK (2001) Impulse to the active indole-3-acetic acid pool: de novo synthesis, conjugate hydrolysis, and indole-3-butyric acid oxidation. J Plant Growth Regul 20:198–216. doi:10.1007/s003440010025
Belmonte MF, Donald G, Reid DM, Yeoung E, Stassolla C (2005) Alterations of the glutathione redox state improve apical meristem structure and somatic embryo quality in white spruce (Picea glauca). J Exp Bot 56:2355–2364. doi:10.1093/jxb/eri228
Benson EE, Lynch PT, Jones J (1992) Variation in free radical damage in rice cell suspensions with different embryogenic potentials. Planta 188:296–305. doi:10.1007/BF00192795
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Bueno P, Piqueras A, Kurepa J, Savouré A, Verbruggen N, Van Montagú M, Inzé D (1998) Expresión of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures. Plant Sci 138:27–34. doi:10.1016/S0168-9452(98)00154-X
Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142:775–787. doi:10.1104/pp.106.085506
Chichiricco G, Grilli-Caiola M (1987) In vitro development of partenocarpic fruits of Crocus sativus L. Plant Cell Tissue Organ Cult 11:75–78. doi:10.1007/BF00036578
De Gara L, de Pinto MC, Arigoni D (1997) Ascorbate síntesis and ascorbate peroxidase activity during the early stage of wheat germination. Physiol Plant 100:894–900. doi:10.1034/j.1399-3054.1997.1000415.x
Deighton N, Magil WJ, Bremner DH, Benson EE (1997) Malondialdehyde and 4-hydroxy-2-nonenal in plant tissue cultures: LC-MS determination of dinitrophenylhydrazone derivatives. Free Radic Res 27:255–257. doi:10.3109/10715769709065764
Dutta Gupta S, Datta S (2003) Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regeneration. Biol Plant 47:179–183. doi:10.1023/B:BIOP.0000022248.62869.c7
Escribano J, Piqueras A, Medina J, Rubio A, Alvarez-Orti M, Fernandez JA (1999) Production of cytotxic proteoglycan using callus cultures of saffron corms (Crocus sativus L.). J Biotechnol 73:53–59. doi:10.1016/S0168-1656(99)00125-X
Fereol L, Chovelon V, Causse S, Michaux-Ferriere N, Kahane R (2002) Evidence of a somatic embryogenesis process for plant regeneration in garlic (Allium sativun L.). Plant Cell Rep 21:197–203. doi:10.1007/s00299-002-0498-0
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071. doi:10.1111/j.1365-3040.2005.01327.x
George PS, Visvanath S, Ravishankar GA, Venkatamaran LV (1992) Tissue culture of saffron (Crocus sativus L.): somatic embryogenesis and shoot regeneration. Food Biotechnol 6(3):217–223
Grilli-Caiola M (2005) Embryo origin and development in Crocus sativus L. Plant Biosyst 139:335–343. doi:10.1080/11263500500340763
Hernández JA, Campillo A, Alarcón JJ, Sevilla F (1999) Response of antioxidant system and leaf water relations to NaCl stress in pea plants. New Phytol 141:241–251. doi:10.1046/j.1469-8137.1999.00341.x
Hernández JA, Ferrer MA, Jiménez A, Ros-Barceló A, Sevilla F (2001) Antioxidant systems and O ·-2 /H2O2 production in the apoplast of Pisum sativum L. leaves: its relation with NaCl-induced necrotic lesions in minor veins. Plant Physiol 127:817–831.
Imin N, Nizamidin M, Daniher D, Nolan KE, Rose RJ, Rolfe BG (2005) Proteomic analysis of somatic embryogenesis in Medicago truncata. Explant cultures grown under 6-benzylaminopurine and 1-naphthaleneacetic acid treatments. Plant Physiol 137:1250–1260. doi:10.1104/pp.104.055277
Jiménez A, Hernández JA, Del Río LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114:275–284
Kairong C, Gengsheng X, Xinmin L, Gengmei X, Yafu W (1999) Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarun L. Plant Sci 146:9–16. doi:10.1016/S0168-9452(99)00087-4
Karamian R, Ebrahimzadeh H (2001) Plantlet regeneration from protoplast derived embryogenic calli of Crocus cancellatus. Plant Cell Tissue Organ Cult 68:115–121. doi:10.1023/A:1010661620753
Libik M, Konieczny R, Pater B, Slesak I, Miszalski Z (2005) Differences in the activities of some antioxidant enzymes and H2O2 content during rhizogenesis and somatic embryogenesis in callus cultures of the ice plant. Plant Cell Rep 23:834–841. doi:10.1007/s00299-004-0886-8
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein. J Biol Chem 244:6049–6055
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Obert B, Benson E, Millan E, Prêtóvá A, Bremmer DH (2005) Moderation of morphogenic and oxidative stress responses in flax in vitro cultures by hydroxynonenal and desferroxiamine. J Plant Physiol 162:537–547. doi:10.1016/j.jplph.2004.06.002
Palma J, Garrido M, Rodriguez M, Del Rio LA (1991) Peroxisome proliferation and oxidative stress mediated by activated oxygen species in plant species. Arch Biochem Biophys 287:68–74. doi:10.1016/0003-9861(91)90389-Z
Pasternak TP, Prinsen E, Ayaydin F, Miskolezi P, Potters G, Asard H, Van Onkelen HA, Dutis D, Feher A (2002) The role of auxin, Ph, and stress in the activation of embryogenic cell division in leaf protoplasts-derived cells of alfalfa. Plant Physiol 129:1807–1819. doi:10.1104/pp.000810
Pasternak TP, Potters G, Caubergs R, Jansen MAK (2005) Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ and cellular level. J Exp Bot 58:1991–2001. doi:10.1093/jxb/eri196
Piqueras A, Han BH, Escribano J, Rubio C, Hellín E, Fernandez JA (1999) Development of cormogenic nodules and microcorms by tissue culture, a new tool for the multiplication and genetic improvement of saffron. Agronomie 19:603–610. doi:10.1051/agro:19990704
Quiroz-Figueroa F, Mendez-Zeel M, Sánchez-Teyer F, Rojas-Herrera R, Loyola-Vargas VM (2002) Differential gene expression in embryogenic and non embryogenic clusters form cell suspension cultures of Coffea Arabica. J Plant Physiol 159:1250–1267. doi:10.1078/0176-1617-00878
Radojevic J, Subotic A (1992) Plant regeneration of Iris setosa Pall through somatic embryogenesis and organogenesis. J Plant Physiol 139:690–696
Sage DO, Lynn J, Hammat N (2000) Somatic embryogenesis in Narcisus Pseudonarcissus cvs. Golden Harvesat and St Keverne. Plant Sci 150:209–216. doi:10.1016/S0168-9452(99)00190-9
Sparkes IA, Brandizzi F, Slocombe SP, El-Shami M, Hawes C, Baker A (2003) An Arabidopsis pex10 null mutant is embryo lethal, implicating peroxisomes in an essential role during plant embryogenesis. Plant Physiol 133:1809–1819. doi:10.1104/pp.103.031252
Stefaniak B (1994) Somatic embryogenesis and plant regeneration of gladiolus (Gladiolus Hort). Plant Cell Rep 3:386–389
Stewart RC, Bewley D (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol 65:245–246. doi:10.1104/pp.65.2.245
Thibaud-Nissen F, Shealy RT, Khanna A, Vodkin LO (2003) Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiol 132:118–136. doi:10.1104/pp.103.019968
Thorpe TA, Stasolla C (2001) Somatic embryogenesis. In: Bhojwani SS, Soh WY (eds) Current trends in the embryology of angiosperms. Kluwer Publishers, Dordrecht, pp 279–336
Tian M, Gu Q, Zhu M (2003) The involvement of hydrogen peroxide and antioxidant enzymes in the process of shoot organogenesis of strawberry callus. Plant Sci 165:701–707. doi:10.1016/S0168-9452(03)00224-3
van Breusegem FM, Vranova E, Dat JF, Inzé D (2001) The role of active oxygen in plant signal transduction. Plant Sci 161:405–414. doi:10.1016/S0168-9452(01)00452-6
von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249. doi:10.1023/A:1015673200621
Wang Y, Jeknic Z, Ernst RC, Chen TH (1999) Efficient plant regeneration from suspension-cultured cells of tall bearded iris. HortScience 34(4):730–735
Weber H (2000) Fatty acid-derived signals in plants. Trends Plant Sci 7:217–224. doi:10.1016/S1360-1385(02)02250-1
Weissiger RA, Fridovich I (1973) Superoxide dismutase: organelle specificity. J Biol Chem 248:3582–3592
Yeung EC (1995) Structural and developmental patterns in somatic embryogenesis. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer Publishers, Dordrecht, pp 205–247
Yeung EC, Law SK (1987) Serial sectioning techniques for a modified LKB historesin. Stain Technol 62:147–153
Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S (2000) Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol 123:223–234. doi:10.1104/pp.123.1.223
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blazquez, S., Olmos, E., Hernández, J.A. et al. Somatic embryogenesis in saffron (Crocus sativus L.). Histological differentiation and implication of some components of the antioxidant enzymatic system. Plant Cell Tiss Organ Cult 97, 49–57 (2009). https://doi.org/10.1007/s11240-009-9497-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9497-y