Abstract
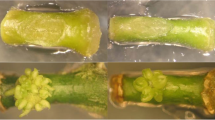
Ilumination did not affect the pathway of shoot regeneration at the cut edges of epicotyl explants of Troyer citrange (Moreira-Dias et al. 2000, 2001), but signigficantly affected the number of developed shoots and the response to exogenous cytokinins. Shoot regeneration at the apical end occurred through a direct organogenic pathway without callus formation. For explants incubated in the light, this regeneration did not require cytokinin addendum, but the number of shoots formed was significantly increased by benzyl adenine, but not by zeatin or kinetin. Incubation in the dark almost suppressed shoot formation at the apical end. The addition of benzyl adenine or kinetin, but not of zeatin, restored shoot formation in the dark to the value obtained in the light. At the basal end of the explants shoot regeneration occurred through an indirect organogenic pathway after the formation of a primary callus. In explants incubated in the light, callus formation and shoot growth was supported by a low (0.5–1 mg l−1) benzyl adenine concentration and by zeatin. Kinetin did not support callus growth. Shoot formation was higher in the presence of benzyl adenine (0.5–1 mg l−1) than of zeatin, but was inhibited by a high (5 mg l−1) benzyl adenine concentration. Incubation in the dark increased callus growth and shoot formation at the basal cut as compared to explants incubated in the light. The three cytokinins tested supported callus growth and shoot formation in the dark, zeatin being the most effective and kinetin the least. In terms of number of shoots developed, the optimum cytokinin addendum depended on the pathway of organogenesis and the conditions of incubation. The maximum number of shoots developed at the apical end was obtained when the incubation was performed in the light in the presence of benzyl adenine. At the basal end, the optimal conditions were incubation in the dark in the presence of zeatin. It was not always possible to define an optimal cytokinin concentration as the curve concentration/response varied from experiment to experiment, which seemed unrelated to the endogenous cytokinin concentration in the explants.







Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzylaminopurine
- K:
-
Kinetin
- Z:
-
Zeatin
References
Al-Bahrany AM (2002) Effect of phytohormones on in vitro shoot multiplication and rooting of lime Citrus aurantifolia (Christm.) Swing. Sci Hortic 95:285–295
Barlass M, Skene KGM (1982) In vitro plantlet formation from Citrus species and hybrids. Sci Hortic 17:333–341
Bordón Y, Guardiola JL, García-Luis A (2000) Genotype affects the morphogenic response in vitro of epicotyl segments of Citrus rootstocks. Ann Bot 86:159–166
Durán-Vila N, Gogorcena Y, Ortega V, Ortiz J, Navarro L (1992) Morphogenesis and tissue culture in sweet orange (Citrus sinensis (L.) Osb.): effect of temperature and photosynthetic radiation. Plant Cell Tissue Organ Cult. 29:11–18
Edris MH, Burger DW (1984) In vitro propagation of Troyer citrange from epicotyl segments. Sci Hortic 23:159–162
García-Luis A, Bordón Y, Moreira-Dias JM, Molina RV, Guardiola JL (1999) Explant orientation and polarity determine the morphogenic response of epicotyl segments of Troyer citrange. Ann Bot 84:715–723
García-Luis A, Molina RV, Varona V, Castelló S, Guardiola JL (2006) The influence of explant orientation and contact with the medium on the pathway of shoot regeneration in vitro in epicotyl cuttings of Troyer citrange. Plant Cell Tissue Organ Cult 85:137–144
Gmitter FC, Grosser GW, Moore GA (1992) Citrus. In: Hammerschlag FA, Ritz RE (eds) Biotechnology of perennial fruit crops. CAB International, Wallingford, UK, pp 335–369
Goh CJ, Sim GE, Morales CL, Loh CS (1995) Plantlet regeneration through different morphogenic pathways in pommelo tissue culture. Plant Cell Tissue Organ Cult 43:301–303
Hassanein AM, Azooz MM (2003) Propagation of Citrus reticulata via in vitro seed germination and shoot cuttings. Biol Plant 47:173–177
Huang T, Peng SL, Dong GF, Zhang LY, Li GG (2002) Plant regeneration from leaf-derived callus in Citrus grandis (pummelo): effects of auxins in callus induction medium. Plant Cell Tissue Organ Cult 69:141–146
Joy IV-RW, Thorpe TA (1999) Shoot morphogenesis: structure, physiology, biochemistry and molecular biology. In: Soh WY, Bhojwani SS (eds) Morphogenesis in plant tissue cultures. Kluwer, Dordrecht, The Nederlands, pp 171–215
Kobayashi AK, Bespalhop JC, Pereira LFP, Vieira LGE (2003) Plant regeneration of sweet orange (Citrus sinensis) from thin sections of mature stem segments. Plant Cell Tissue Organ Cult 74:99–102
Moore GA, Jacono CC, Neidigh JL, Lawrence SD, Kline K (1992) Agrobacterium-mediated transformation of Citrus stem segments and regeneration of transgenic plants. Plant Cell Rep 11:238–242
Moreira-Dias JM, Molina RV, Bordón Y, Guardiola JL, García-Luis A (2000) Direct and indirect shoot organogenic pathways in epicotyl cuttings of Troyer citrange differ in hormone requirements and in their response to light. Ann Bot 85:103–110
Moreira-Dias JM, Molina RV, Guardiola JL, García-Luis A (2001) Daylength and photon flux density influence the growth regulator effects on morphogenesis in epicotyl segments of Troyer citrange. Sci Hortic 87:275–290
Pérez-Molphe-Balch E, Ochoa-Alejo N (1997) In vitro plant regeneration of Mexican lime and mandarin by direct organogenesis. HortScience 32:931–934
Raj-Bhansali R, Arya HC (1978) Shoot formation in stem and root callus of Citrus aurantifolia (Christm) “Swingle”, grown in cultures. Current Sci 47:775–776
Sim GE, Goh CJ, Loh CS (1989) Micropropagation of Citrus mitis Blanco. Multiple bud formation from shoot and root explants in the presence of 6-benzylaminopurine. Plant Sci 52:203–210
Veltcheva M, Svetleva D, Petkova Sp, Perl A (2005) In vitro regeneration and genetic transformation of common bean (Phaseolus vulgaris L). Problems and progress. Sci Hortic 107:2–10
Acknowledgements
This research was funded by Conselleria de Agricultura, Pesca y Alimentación, Generalitat Valenciana (Grant GV-CAPA00–11), and Consellería d’Empresa, Universitat i Ciència (Grupos 04/059). We thank Dr. J. Forner (IVIA, Valencia, Spain) for providing the seeds. These results are part from a M.Sc. Thesis by S.C. for the degree of Ingeniero Agrónomo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molina, R.V., Castelló, S., García-Luis, A. et al. Light cytokinin interactions in shoot formation in epicotyl cuttings of Troyer citrange cultured in vitro. Plant Cell Tiss Organ Cult 89, 131–140 (2007). https://doi.org/10.1007/s11240-007-9221-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-007-9221-8