Abstract
Acute myeloid leukemia (AML) is a heterogeneous hematological malignancy, and is one of the triggers of DIC, the latter is an essential factor in the early death of patients with AML. However, the timely identification of DIC remains a challenge. The Chinese DIC Scoring System (CDSS) is a common consensus widely used in China; but, there are few reports on its application in patients with AML. We undertake this retrospective cohort study to investigate the association between CDSS score and 60-day mortality. CDSS scores were evaluated after admission. The outcome was all-cause 60-day mortality. Multivariate Cox regression analyses were performed to calculate the adjusted hazard ratio (HR) and the corresponding 95% confidence interval (CI). Survival curves were plotted by Kaplan–Meier and log-rank analyses. Subgroup analyses were stratified by relevant effect covariates. A total of 570 consecutive patients with primary AML were included. We found an association between a 39% increase in 60-day mortality and a 1 point increase in CDSS score (HR = 1.39, 95% CI 1.25–1.54), which was associated with a 189% increase in 60-day mortality in CDSS scores ≥ 6 compared with that in the CDSS scores < 6 (HR = 2.89, 95% CI 1.91–4.38). After adjusting for all potential con-founders, a 27% and a 198% increase were observed (HR = 1.27, 95% CI 1.01–1.61; HR = 2.98, 95% CI 1.24–7.19), respectively. There is association between 60-day mortality and CDSS score in patients with AML. These findings may help hematologists in making informed treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
The CDSS score may be closely associated with 60-day mortality in patients with primary non-APL AML.
-
These results, which are of great clinical significance in patients with CDSS scores ≥ 6, suggest that hematologists should quickly start anti-DIC and anti-leukemia treatments.
Introduction
Acute myeloid leukemia (AML), one of the most common type of hematological malignancies in adults, is a heterogeneous clonal myeloid neoplasm characterized by maturation arrest of hematopoietic progenitor cells, leading to uncontrolled blast proliferation [1,2,3]. Abnormal differentiation of myeloid cells results in high levels of immature malignant cells and low levels of healthy hematopoietic elements. Cytopenia causes clinical manifestations, with symptoms of anemia (e.g., dyspnea and fatigue), thrombocytopenia (hemorrhage), and neutropenia (infections), which are usually present at the time of diagnosis and accompanied by treatment [4].
The early mortality was 21.0–37.5% reported in previous studies [5,6,7], although hematologists have taken much effort into reducing 60-day mortality, commonly known as early death, defined as death from any cause within 60 days of hospitalization with AML [7, 8],it remains a vital clinical problem to be solved [9]. Disseminated intravascular coagulation (DIC) is a systemic activation of the coagulation system that results in microvascular thrombosis and, simultaneously, potentially life-threatening hemorrhage attributed to the consumption of platelets and coagulation factors. Underlying conditions, including hematological malignancies and infection, are responsible for the initiation and propagation of DIC[10, 11]. Patients with AML are prone to co-infection, both AML and infection are trigger factors of DIC. DIC, considered a clinical laboratory diagnosis by thrombosis and hemostasis specialists, is complicated [12]. Patients with DIC tend to stay in critical condition and have high mortality rates. It is still a challenging task requiring professional experience to diagnose DIC in patients with AML accurately [13]. In China, the latest edition of the consensus of Chinese experts on DIC diagnosis published in 2017 has been widely accepted by physicians and investigators caring for DIC patients [14, 15]. However, the association between the Chinese Disseminated Intravascular Coagulation Scoring System (CDSS) score and 60-day mortality is uncertain, and only limited data are available on the application of the CDSS for diagnosing DIC in patients with non-APL AML [16].This study aimed to investigate the association between CDSS scores and 60-day mortality in primary AML.
Methods
This is a cohort study based on retrospectively collected consecutive data from patients of all ages, verified primary non-APL AML at the Affiliated Ganzhou Hospital of Nanchang University (Jiangxi Province, China) from January 2013 to July 2022. All individuals in this study underwent bone marrow (BM) aspiration and had confirmed AML diagnosis based on more than two methods of morphological, immunophenotype, cytogenetic, and molecular analysis (MICM), according to the World Health Organization (WHO) classification system (version 2016) [17] The patients were classified with genomic risk category, which were quired by karyotype analysis (G-banding), fluorescence in situ hybridization (FISH) and next generation sequencing (NGS) [1, 18]. This study followed the principles of the Declaration of Helsinki and was approved (Ethics number: 202005) by the Ethics Review Board of the Affiliated Ganzhou Hospital of Nanchang University [19].
Patients diagnosed with acute promyelocytic leukemia (APL) were excluded, due to different management and treatment for APL and non-APL AML [20, 21]. Patients with a history of other hematological malignancies, such as chronic myeloid leukemia (CML), myelodysplastic syndrome (MDS), and myelodysplastic syndrome/ myeloproliferative neoplasms (MDS/MPN), were excluded due to secondary AML, read our previous report in brief [19]. Patients with the following criteria were also excluded: thrombotic thrombocytopenic purpura (TTP) based on clinical history, examination and routine laboratory parameters, having anti-phospholipid syndrome (APS) based on at least one of the clinical and one of the laboratory criteria, liver cirrhosis classified as Child–Pugh grade C (score 10 or higher). These primary diseases might lead to abnormal platelet (PLT), D-dimer (DD), or fibrinogen (Fg) and could affect CDSS scores. Mixed phenotype acute leukemia (MPAL) was also excluded due to the strict sense of patients with non-AML [17].
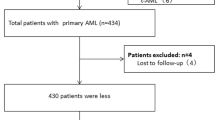
In addition, patients did not undergo complete CDSS test items or were lost to follow-up were excluded; all remaining patients were included in the study with or without chemotherapy (Fig. 1). None of the patients underwent bone marrow transplantation 60 days after admission. The final cohort included 570 patients with AML classified into three subtypes: AML-M2, AML-M5, and the other subgroup. The other subgroup included 127 patients classified as follows: AML-M0 (n = 5), AML-M1 (n = 40), AML-M4 (n = 68), AML-M6 (n = 4), and AML-M7 (n = 10). (Fig. 1).
CDSS score
The latest CDSS was established in 2017 by a consensus of Chinese experts on the diagnosis and treatment of DIC [14]. According to this consensus, DIC is diagnosed when a patient with hematologic malignancies has abnormal laboratory findings [14].This consensus assigns quantitative weights to individual laboratory and clinical parameters for DIC diagnosis. The CDSS criteria includes following five rules. (1) A primary disease that causes DIC, hematologic malignancy e.g.is present, 2 points. (2) Prolongation of PT and APTT: PT < 3S and APTT < 10 S,0 point; PT ≥ 3S and APTT ≥ 10 S,1 point; PT > 6S, 2 points. (3)D-dimer level: < 5 μg/mL, 0 point; ≥ 5 μg/mL, 2 points; ≥ 9 μg/mL, 3 points. (4) Fibrinogen level: ≥ 1.0 g/L,0 point; < 1.0 μg/mL, 1point. (5) Platelet counts: > 50 × 109/L,0 point; Platelet counts < 50 × 109/L,1 point; Diagnosis of DIC is CDSS score ≥ 6 points for AML Patients (Table S1). All, except PLT count, were measured using the blood coagulation analyzer Sysmex CS-5100 System TM (Siemens Healthcare Diagnostics, Erlangen, Germany) with the corresponding diagnostic reagents kit (Siemens Healthcare Diagnostics Products, Germany, Marburg, Germany). and the reference interval for PT was 9–13 s, APTT 20–40 s, Fib 2–4 g/L, and DD 0–0.55 mg/L, in accordance with the national standard. Commercially available control materials for internal quality control (IQC) (Siemens Healthcare Diagnostics Products, Marburg, Germany) were used to test blood samples daily. PLT counts were obtained from routine blood samples, measured using a Sysmex XE-2100 or XN-1000 s. All five items, including the CDSS score, were included in external quality assessment (EQA) activities organized by the National Center for Clinical Laboratories (NCCL) every year, and criteria for feedback reports were fulfilled during this study. All data (except PLT) from this study, including CDSS and other items/parameters, were the first test results of patients after admission for AML. If the CDSS score of the patients was ≥ 6, they were diagnosed with overt DIC, and if the CDSS score was 5 points with PLT ≥ 50 × 109/L for the first time, another two PLT count were required for monitoring; if the PLT decreased by 50% within 48 h, the CDSS score would be calculated repeatedly.
Outcomes
The primary endpoint and outcome of interest were death within 60 days of admission. The data collectors for clinical information at the first diagnosis were blinded to the survival data.
Data collection
Data, including survival status, were collected from the electronic medical record system or follow-up telephone calls. Baseline examinations included FAB subtype, genomic risk category, bleeding and thrombosis, pulmonary infection, blood and bone marrow (BM) parameters. The biomarkers included white blood cell (WBC) count, PLT count, prothrombin time (PT), activated partial thromboplastin time (APTT), DD, Fg, creatine kinase isoenzyme-MB (CK-MB), myoglobin (Myo), antithrombin (AT), albumin (Alb), creatinine (Crea), glucose (Glu), serum ferritin (SF), and BM blast. All laboratory data were focused on measurements performed within the first 48 h of admission to reduce the probability that serum biomarker levels were affected by anti-leukemia and transfusion therapy. These parameters comprise routine testing, which physicians commonly use to evaluate a patient’s physical condition. The chemotherapy was completed within 60 days.
Statistical analysis
This study aimed to observe the impact of CDSS score on 60-day mortality in patients with AML. The patients were divided into two groups based on their CDSS scores. A descriptive analysis was applied to all patients. Continuous data were expressed as mean and standard deviation (SD) or median and interquartile range (quartile 1–quartile 3 [interquartile range (IQR)], as appropriate. Categorical variables were expressed as proportions (%). Variables were compared using the chi-square test (categorical variables), one-way ANOVA (normal distribution), and Kruskal–Wallis (skewed distribution) tests [19].
Multivariate Cox regression analysis was used to assess the independent association between CDSS score and 60-day mortality. In the analysis, confounding factor is an important issue, we performed some different statistical models to verify the results’ stability. In the final model, we adjusted the factors basing the following two rules. (1) For univariate analysis, we adjusted for variables, of which the p values were less than 0.1(Table S2). (2) For multivariate analysis, variables were chosen on the basis of previous findings and clinical constraints. Dummy variables were used to indicate the missing covariance values, if the missing data variables were greater than 10%. Considering the strong heterogeneity of WBC in leukemia, WBC were used as a dichotomous variable to adjust in multivariate Cox regression analysis.
Survival curves were plotted by Kaplan–Meier and log-rank analyses. Subgroup analyses were stratified by relevant effect covariates. Analyses were stratified according to the results of the univariate analysis (P-value < 0.1) and basis of previous findings and clinical constraints, including sex, age, WBC, Glu, ALB, chemotherapy and bleeding, to examine the effect of these factors on the above associations. The likelihood ratio test was used to assess effect modification according to prespecified subgroups using interaction terms between subgroup indicators and CDSS scores. Interactions across subgroups were tested using the likelihood ratio test. All analyses were performed using R 3.3.2 (http://www.R-project.org, The R Foundation) and Free Statistics (version 1.5). Differences with a two-sided P < 0.05 were considered statistically significant [22].
Results
Baseline characteristics of the study participants by categories of CDSS score
Of the 706 newly diagnosed patients with AML during the study period, 570 ones were eligible for analysis (Fig. 1). The descriptive characteristics of the eligible study population with a CDSS < 6 and ≥ 6 are reported in Table 1. Patient age was 51.3 (SD 20.4) years (range, 1–94 years), 306 (53.7%) were male, and 137 (24.0%) suffered from 60-day mortality. In addition, 342 (60.1%) patients received combined chemotherapy, and 92 (16.2%) patients single chemotherapy drugs. The combined chemotherapy regimens were a combination of cytarabine and anthracycline “7 + 3” or a combination of cytarabine and the other, 7 patients were treated with the combination of cytarabine and venetoclax. Single chemotherapy meant patients only used decitabine or hydroxyurea [23]. The median CDSS score level was 3.4 ± 1.4 points (range, 0–9 points). 59 (10.4%) cases had CDSS scores ≥ 6 points. The 60-day mortality of patients with CDSS score ≥ 6 was as high as 47.5%, significantly higher than those < 6 at 21.3% (P < 0.001).
Additionally, among CDSS laboratory integral items, the PLT lower than 50 × 109/L had the highest abnormal rate with 61.1% (348 cases), followed by the D-dimer (≧5.0 mg/L) 24.2% (138 cases). There were 8 cases (1.4%) with prolongation of APTT (≧10 S) and 51 cases (8.9%) that of PT prolongation (≧3 S). Among 59 patients with overt DIC, the abnormality rate of DD was as high as 96.6% (57 cases, including DD ≥ 5 mg/L in 11 cases and ≥ 9 mg/L in 46 cases), followed by PLT < 50 × 109/L in 93.2% (55 cases), prolongation of PT (≥ 3S), 40.7% (24 cases), Fg(< 1.0 g/L) 18.6%(11 cases), prolongation of APTT (≥ 10S) 4.1% (4 cases).
We evaluated the cohort with ISTH creteria, and found there were 89 (15.6%) patients who had elevated ISTH scores (≥ 5) diagnostic of DIC, with early mortality 42.7%, among those patients there were 58 ones with CDSS score ≥ 6, 28 patients suffered from early death. The morbidity of DIC was 10.4% according to creteria of CDSS, which was lower than that of ISTH one, but the mortality was 47.5%, higher than the former.
Association between CDSS score and 60-day mortality
In the extended multivariate Cox models (Table 2), we observed that the hazard ratios (HRs) of the CDSS score (increase per 1 point) were consistently significant in all three models (HRs range 1.26–1.39). Patients with a CDSS score ≥ 6 (overt DIC) had a 189% higher risk of 60-day mortality than patients with a CDSS score < 6 (non-overt DIC) (HR = 2.89, 95% confidence interval (CI) 1.91–4.38). After adjusted for all covariates, results showed a 198% higher 60-day mortality rate in patients with a CDSS score ≥ 6 (HR = 2.98, 95% CI 1.24–7.19, model III, Table 2) than in those with that < 6. The covariates were confirmed after a univariate analysis of risk factors with a P-value ≤ 0.027 (Table S2). Dummy variables were used for serum ferritin due to its missing data was14%. In this observation population, WBC ranged from 0.29 × 109/L to 540.52 × 109/L, with a median of 19.7 × 109/L. We divided WBC into two groups with 20 × 109/L, then used binary WBC as one of the adjusted variables of multivariate Cox regression analysis. We also analyzed the data using the International Society on Thrombosis and Hemostasis (ISTH) criteria and obtained similar results (Table S3). The Kaplan–Meier curve showed that mortality was higher by day 60 in patients with a CDSS score ≥ 6 (log-rank test: P < 0.0001, Fig. 2).
Subgroup analyses
Analyses and interactive analyses were stratified according to confounders to detect whether the association between CDSS score and 60-day mortality of AML was present in different subgroups. The confounders that were confirmed after a univariate analysis of risk factors (Table S2) and previous findings and clinical constraints, included age, sex, WBC, Glu, ALB, chemotherapy and bleeding (Fig. 3). No significant interaction was observed in any subgroups (P-value for interaction > 0.05).
Stratified analyses of the association between CDSS score and 60-day mortality according to baseline characteristics. Note: The p-value for interaction represents the likelihood of interaction between the variable and the CDSS score. WBC was entered as a categorical variable. The event is so less in thrombosis subgroup, that it was not shown. Adjusts for: sex + age + WBC + AT + Alb + Myo + Glu + chemotherapy + bleeding or thrombosis; CDSS Chinese DIC scoring system, DIC disseminated intravascular coagulation, WBC white blood cell, Glu glucose, Alb albumin, HR hazard ratio, CI confidence interval
Cause of 60-day mortality
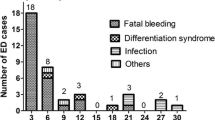
There were 137 patients suffered from 60-day mortality, the main causes were organ failure, hemorrhage or Thrombosis, infection, particular pulmonary infection (Table 3).
Discussion
This paper reports a cohort study on the association between CDSS score and 60-day mortality in patients with primary AML. In this real-world study, we evaluated Chinese patients with primary AML using the CDSS criteria and found that 10.4% of patients met the overt DIC criteria (CDSS ≥ 6), similar to a previous report [16]. Among patients with overt DIC, the 60-day all-cause death rate was as high as 47.5%, over twice that of patients with non-overt DIC (21.3%). The risk increased with an increase in the CDSS score, independent of sex, age, and other confounders. The results remained robust with no or gradual adjustments. These findings illustrate that physicians should pay more attention to DIC screening in patients with primary AML and start timely intervention and early treatment to improve patient outcomes. We also noted that patients with elevated leukocyte counts (WBC ≥ 20 × 109/L) were more likely to develop DIC, as was AML-M5, as previously reported [24]. Abnormal frequencies of DD and PLT are higher in CDSS laboratory integral terms, so special attention should be paid to these two items in the diagnosis of AML secondary DIC. It has long been known that PT and APTT are normal in more than half of AML patients with DIC [12].APTT was added to the CDSS integration system to improve the sensitivity of DIC. In our study, 40.7% (24 cases) of overt DIC patients had prolongation of PT (≥ 3S) or 4.1% (4 cases) prolongation of APTT ≥ 10 s, respectively. The results are consistent with those of previous reports; however, the abnormal APTT rate is much lower among those patients. In a prospective study, Wu et al. explored the association between CDSS scores and 28-day all-cause mortality in 753 patients (including sepsis/severe infection, trauma or surgery, and solid tumors, among others). Their results demonstrated that the CDSS DIC score was an independent predictor of mortality [25]. However, they neither included patients with AML nor adjusted for confounders. Additionally, we observed that the mean age of patients with AML was 51.8 years, much younger than that of the European population (65 years) [4].
In CDSS criteria, platelet is different between patients with Hematologic neoplasms and Non-Hematologic neoplasms,compared to ISTH criteria, and D-dimer included clear concentration criteria, while ISTH criteria included judgement of no, slightly elevated and significantly elevated. So, These refinement may be easier for clinicians. In multivariate Cox regression, the CDSS and ISTH scores, as continuous or binary variables, HRs and 95% CIs were similar; however, the former HRs were slightly higher and the laboratory items are more widespread in China.
Hematologists usually evaluate the risk of early mortality based on the clinical performance status and laboratory data. However, the definition of early mortality in AML was different; it was defined as death within 60 days from diagnosis or at the start of chemotherapy [7, 8]. A previous study reported an early mortality rate of 21.0–37.5% [5, 7, 8, 26]. Our results, which showed mortality within 60 days after admission was 24.0%, are similar to those of previous studies. Furthermore, 60-day mortality or early mortality remains an essential clinical problem, which is the critical period for successful clinical management of patients with AML; however, the causes are complicated and unknown, even though hematologists have been trying to reduce its risk. Our study differs slightly from previous studies. Most studies only included patients who had received chemotherapy [27, 28]; the others were excluded due to poor conditions [8]. DIC is a common and severe complication of critical conditions and is highly life-threatening [15], it also is one of the most common causes of severe intracerebral or pulmonary hemorrhage, and AML may trigger DIC. A shift in the thinking process to recognize DIC early in its progression is stressed to help treat better these patients in the future [12].
This study had several noteworthy limitations. First, DIC is a dynamic pathological process, so "dynamic monitoring" is essential for DIC diagnosis, especially regarding chemotherapy duration. We collected admission data within 48 h, so the findings of this research only reflect the period before anti-leukemia treatment. Second, although these findings raise questions about potential 60-day mortality, interpretation is limited by the observational study design; therefore, we could not have a direct risk of CDSS score for patients with AML. Third, the data on fibrin degradation products (FDP) were not available for most cases, so we could not adjust FDP in multivariate Cox regression analysis; however, we compared the CDSS score to the ISTH score and found that the hazard ratios (HRs) were similar between the two scoring systems in the crude model or different adjusted models. Furthermore, this is a retrospective study; the data were collected from 2013 to 2021, and the date of death data for some patients was obtained by telephone follow-up and may be biased. To reduce bias, we conducted interviews with at least two or three family members to determine the exact survival time of the patients. Moreover, different batches of experimental reagents may affect the test results of the CDSS project to some degree. To make the values dependable, IQC was required every day to ensure that the results were under control before testing the clinical specimens. We also participated in an external quality assessment (EQA) organized by the NCCL two times per year to ensure the accuracy of the testing results. Fortunately, they satisfied both the control results. Meanwhile, the instrument must undergo calibration twice yearly as part of regular maintenance. Therefore, all the testing results were dependable.
Conclusion
The CDSS score may be closely associated with 60-day mortality in patients with primary AML. These results, which are of great clinical significance in patients with CDSS scores ≥ 6, suggest that hematologists should quickly start anti-DIC and anti-leukemia treatments. However, further research is required to confirm and validate these associations.
Data availability
The raw data required to reproduce these findings cannot be shared at this time, as the data forms part of an ongoing study. However, if necessary, some or all the data generated or used during the study are available from the corresponding author upon request.
References
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N, Gundem G, Van Loo P, Martincorena I, Ganly P, Mudie L, McLaren S, O’Meara S, Raine K, Jones DR, Teague JW, Butler AP, Greaves MF, Ganser A, Dohner K, Schlenk RF, Dohner H, Campbell PJ (2016) Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med 374(23):2209–2221
Angenendt L, Hilgefort I, Mikesch JH, Schluter B, Berdel WE, Lenz G, Stelljes M, Schliemann C (2021) Magnesium levels and outcome after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Ann Hematol 100(7):1871–1878
Di Genua C, Nerlov C (2021) To bi or not to bi: Acute erythroid leukemias and hematopoietic lineage choice. Exp Hematol 97:6–13
Ferrara F, Schiffer CA (2013) Acute myeloid leukaemia in adults. The Lancet 381(9865):484–495
Sorror ML, Storer BE, Fathi AT, Gerds AT, Medeiros BC, Shami P, Brunner AM, Sekeres MA, Mukherjee S, Pena E, Elsawy M, Wardyn S, Whitten J, Moore R, Becker PS, McCune JS, Appelbaum FR, Estey EH (2017) Development and Validation of a Novel Acute Myeloid Leukemia-Composite Model to Estimate Risks of Mortality. JAMA Oncol 3(12):1675–1682
Bhatt VR, Shostrom V, Giri S, Gundabolu K, Monirul Islam KM, Appelbaum FR, Maness LJ (2017) Early mortality and overall survival of acute myeloid leukemia based on facility type. Am J Hematol 92(8):764–771
Ho G, Jonas BA, Li Q, Brunson A, Wun T, Keegan THM (2017) Early mortality and complications in hospitalized adult Californians with acute myeloid leukaemia. Br J Haematol 177(5):791–799
Krug U, Röllig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M, Thiede C, Kramer M, Braess J, Spiekermann K, Haferlach T, Haferlach C, Koschmieder S, Rohde C, Serve H, Wörmann B, Hiddemann W, Ehninger G, Berdel WE, Büchner T, Müller-Tidow C (2010) Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. The Lancet 376(9757):2000–2008
Walter RB, Estey EH (2020) Selection of initial therapy for newly-diagnosed adult acute myeloid leukemia: limitations of predictive models. Blood Rev 44:100679
Adelborg K, Larsen JB, Hvas AM (2021) Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol 192(5):803–818
Gando S, Levi M, Toh CH (2016) Disseminated intravascular coagulation. Nat Rev Dis Primers 2:16037
Thachil J (2019) The Elusive Diagnosis of Disseminated Intravascular Coagulation: Does a Diagnosis of DIC Exist Anymore? Semin Thromb Hemost 45(1):100–107
Paterno G, Palmieri R, Forte V, Bonanni F, Guarnera L, Mallegni F, Pascala MR, Elisa B, Moretti F, Savi A, Maurillo L, Buccisano F, Venditti A, Del Principe MI (2022) PB1835: the ISTH-DIC score predicts 30-days outcome in non-M3 acute myeloid leukemia patients. Hemasphere 6(Suppl):1715–1716
Thrombosis and Hemostasis Group, Hematology Society of Chinese Medical Association (2017) Consensus of Chinese experts on diagnosis of disseminated intravascular coagulation (version 2017). Zhonghua Xue Ye Xue Za Zhi 38(5):361–363
Luo L, Wu Y, Niu T, Han Y, Feng Y, Ding Q, Huang R, Zhang X, Feng J, Hou M, Peng J, Li Y, Zhou Y, Su L, Yang L, Zhou Z, Xue F, Gu J, Zhu T, Wang X, Deng J, Mei H, Hu Y (2019) A multicenter, prospective evaluation of the Chinese Society of Thrombosis and Hemostasis Scoring System for disseminated intravascular coagulation. Thromb Res 173:131–140
Wang TF, Makar RS, Antic D, Levy JH, Douketis JD, Connors JM, Carrier M, Zwicker JI (2020) Management of hemostatic complications in acute leukemia: Guidance from the SSC of the ISTH. J Thromb Haemost 18(12):3174–3183
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405
Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association (2021) Chinese guidelines for the diagnosis and treatment of adult acute myeloid leukemia (not APL) (2021). Zhonghua Xue Ye Xue Za Zhi 42(8):617–623
Xiao Z, Gong R, Chen X, Xiao D, Luo S, Ji Y (2021) Association between serum lactate dehydrogenase and 60-day mortality in Chinese Hakka patients with acute myeloid leukemia: A cohort study. J Clin Lab Anal 35(12):e24049
Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, de Lima M, Fathi AT, Foran JM, Gojo I, Hall AC, Jacoby M, Lancet J, Mannis G, Marcucci G, Martin MG, Mims A, Neff J, Nejati R, Olin R, Percival ME, Prebet T, Przespolewski A, Rao D, Ravandi-Kashani F, Shami PJ, Stone RM, Strickland SA, Sweet K, Vachhani P, Wieduwilt M, Gregory KM, Ogba N, Tallman MS (2021) NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 22021. J Natl Compr Canc Netw 19(1):16–27
Sanz MA, Fenaux P, Tallman MS, Estey EH, Lowenberg B, Naoe T, Lengfelder E, Dohner H, Burnett AK, Chen SJ, Mathews V, Iland H, Rego E, Kantarjian H, Ades L, Avvisati G, Montesinos P, Platzbecker U, Ravandi F, Russell NH, Lo-Coco F (2019) Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood 133(15):1630–1643
Yang Q, Zheng J, Chen W, Chen X, Wen D, Chen W, Xiong X, Zhang Z (2021) Association Between Preadmission Metformin Use and Outcomes in Intensive Care Unit Patients With Sepsis and Type 2 Diabetes: A Cohort Study. Front Med (Lausanne) 8:640785
Yang X, Wang J (2018) Precision therapy for acute myeloid leukemia. J Hematol Oncol 11(1):3
Libourel EJ, Klerk CPW, van Norden Y, de Maat MPM, Kruip MJ, Sonneveld P, Lowenberg B, Leebeek FWG (2016) Disseminated intravascular coagulation at diagnosis is a strong predictor for thrombosis in acute myeloid leukemia. Blood 128(14):1854–1861
Wu Y, Luo L, Niu T, Han Y, Feng Y, Ding Q, Huang R, Zhang X, Feng J, Hou M, Peng J, Li Y, Zhou Y, Su L, Yang L, Zhou Z, Xue F, Gu J, Zhu T, Wang X, Deng J, Mei H, Hu Y (2017) Evaluation of the new Chinese Disseminated Intravascular Coagulation Scoring System in critically ill patients: A multicenter prospective study. Sci Rep 7(1):9057
Oran B, Weisdorf DJ (2012) Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica 97(12):1916–1924
Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, Kim I, Stevens DA, Fiedler W, Pagoni M, Samoilova O, Hu Y, Anagnostopoulos A, Bergeron J, Hou JZ, Murthy V, Yamauchi T, McDonald A, Chyla B, Gopalakrishnan S, Jiang Q, Mendes W, Hayslip J, Panayiotidis P (2020) Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood 135(24):2137–2145
Wang N, Desai A, Ge B, Li W, Jin X, Bai H, Yu K, Ye H (2020) Prognostic value of hypoalbuminemia at diagnosis in de novo non-M3 acute myeloid leukemia. Leuk Lymphoma 61(3):641–649
Acknowledgements
We thank the Health Commission of Jiangxi Province for supporting this research (Project Contract No.202212505). The views expressed are those of the authors. We thank Dr. Jie Liu (Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital) for his help with this revision.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
ZX and YJ designed the study. HZ participated in the statistical analysis and prepared the manuscript. XC, SL, and DX collected clinical data. All authors reviewed and edited the report and approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflict of interest.
Ethical approval
The study was agreed by the Ethics Review Board of the Affiliated Ganzhou Hospital of Nanchang University (Ethics number: 202005), and was approved by the Ethics Review Board of the Affiliated Ganzhou Hospital of Nanchang University. Additionally, we confirm that all methods were performed in accordance with the relevant guidelines and regulations declared in Clinical Chemistry and Laboratory Medicine.
Informed consent
Informed consent was obtained from all individuals included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, H., Chen, X., Xiao, D. et al. Association of CDSS score and 60-day mortality in Chinese patients with non-APL acute myeloid leukemia: a retrospective cohort study. J Thromb Thrombolysis 56, 423–432 (2023). https://doi.org/10.1007/s11239-023-02850-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02850-6