Abstract
Lipoprotein(a) [Lp(a)] is an independent, genetically determined, and causal risk factor for cardiovascular disease. Laboratory data have suggested an interaction of Lp(a) with platelet function, potentially caused by its interaction with platelet receptors. So far, the potential association of Lp(a) with platelet activation and reactivity has not been proven in larger clinical cohorts. This study analyzed intrinsic platelet reactivity before loading with clopidogrel 600 mg and on-treatment platelet reactivity tested 24 h following loading in patients undergoing elective coronary angiography. Platelet reactivity was tested by optical aggregometry following stimulation with collagen or adenosine diphosphate as well as by flow cytometry. Lp(a) levels were directly measured in all patients from fresh samples. The present analysis included 1912 patients. Lp(a) levels ranged between 0 and 332 mg/dl. There was a significant association of rising levels of Lp(a) with a higher prevalence of a history of ischemic heart disease (p < 0.001) and more extensive coronary artery disease (p = 0.001). Results for intrinsic (p = 0.80) and on-clopidogrel platelet reactivity (p = 0.81) did not differ between quartiles of Lp(a) levels. Flow cytometry analyses of expression of different platelet surface proteins (CD41, CD62P or PAC-1) confirmed these findings. Correlation analyses of levels of Lp(a) with any of the tested platelet activation markers did not show any correlation. The present data do not support the hypothesis of an interaction of Lp(a) with platelet reactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Lipoprotein(a) is as risk factor for coronary events.
-
Laboratory data have suggested an interaction of Lp(a) with platelet function.
-
The present data do not support the hypothesis of an interaction of Lp(a) with intrinsic or on dual antiplatelet therapy platelet reactivity but confirms the importance of Lp(a) as risk factor for coronary events.
-
These findings might be important to define the safety of evolving therapeutic options for lowering Lp(a).
Introduction
Lipoprotein(a) [Lp(a)] is an independent and causal risk factor for premature coronary heart disease, myocardial infarction (MI), atherosclerosis, aortic valve stenosis, and stroke [1,2,3,4]. Lp(a) is a LDL-like particle, which consists of an apolipoprotein B-100 disulfide-linked to an apolipoprotein(a) [Apo(a)] [5]. Levels of Lp(a) are primarily genetically determined, have limited variability within a pre-defined range among individuals, and show a wide variance among different populations [2, 6]. Lp(a) appears to be an attractive target for reduction of cardiovascular events since retrospective analyses have suggested that lowering of levels of Lp(a) appears to be associated with a reduced cardiovascular risk [4]. In particular, antisense oligonucleotide therapy seems to be a promising way to lower levels of Lp(a) [7, 8].
The association of Lp(a) with potential anti-fibrinolytic activity is well known, yet the clinical relevance of this has been recently questioned by studies showing no association of genetically determined Lp(a) levels with deep venous thromboses [9]. In addition, several studies have indicated that there might be a potential interaction of Lp(a) with the plasma coagulation cascade and platelet function through its apo(a) component and content of oxidized phospholipids. For example, Lp(a) may activate platelets through the surface receptors PAR-1 and CD36 and in contrast, may prevent latent activation/aggregation through the receptors GPIIb/IIIa and P2Y1 as well as by interacting with platelet activating factor (Fig. 1) [10,11,12,13,14,15,16,17,18,19,20,21,22]. The net effect of these divergent interactions cannot be easily determined in vitro. Therefore, patient cohorts on therapies affecting these pathways are required to assess their clinical relevance. Given the novel therapeutic options for reducing levels of Lp(a), it appears of importance to evaluate this potential interaction to define the safety of lowering Lp(a). Thus, the present study sought to investigate the potential association of Lp(a) levels with platelet activation and on-treatment platelet reactivity in a large clinical cohort.
Potential pathways of interaction of LP(a) with platelet function. Shown is an overview of important platelet receptors with its ligands. Potential receptors by which Lp(a) as ligand could activate (red arrows) or inhibit (blue arrows) platelet aggregation are presented. ADP adenosine diphosphate, ASA acetylsalicylic acid, cAMP Cyclic adenosine monophosphate, COX-1 cyclooxygenase-1, Lyso-PC Lysophosphatidylcholines, OxPL oxidized phospholipids, PAC-1 procaspase-activating compound 1, PAF platelet-activating factor, PLA2 Phospholipases A2, PSGP1 P-selectin glycoprotein ligand 1, SCH530348 Vorapaxar, TRAP/SFLLRN thrombin receptor activating peptide, TxA2 Thromboxan-A2, VASP vasodilator-stimulated phosphoprotein, vWF von willebrand factor
Material and methods
Study population
The present study is a secondary analysis of the prospective EXCELSIOR study (Impact of Extent of Clopidogrel-Induced Platelet Inhibition during Elective Stent Implantation on Clinical Event Rate; ClinicalTrials.gov Identifier: NCT00457236), which investigated platelet reactivity in patients undergoing elective coronary angiography as potential candidates for percutaneous coronary intervention (PCI) [23]. The study was approved by the ethics committee of the University of Freiburg, Germany. Written informed consent was given by all participants.
All patients received a pre-treatment with 600 mg of clopidogrel and were on aspirin before coronary angiography. Patients not on aspirin ≥ 100 mg daily for five days bevor enrolment received an oral loading dose of 400 mg (24.7% of patients, given at mean 3.1 h bevor coronary angiography). Key exclusion criteria were chronic treatment with clopidogrel or ticlopidine or current oral anticoagulation, contraindication to aspirin, clopidogrel or heparin as well as active cancer or terminal renal failure. PCI was performed as previously described [23, 24] and was timed according to the routine schedule of the catheterization laboratory. All patients received a maintenance therapy with clopidogrel (75 mg daily) if coronary intervention was performed together with aspirin (≥ 100 mg) lifelong. Patients were contacted by telephone up to 10 years following enrolment to obtain a complete long-term follow-up. Key clinical endpoints were death of any cause and MI according to the Universal Definition of Myocardial Infarction.
Platelet function studies
Blood samples were drawn before loading with clopidogrel to test intrinsic platelet reactivity and at day 1 following loading 2–4 h after intake of the first maintenance dose of clopidogrel. Tubes containing 3.2% sodium-citrate (Sarstedt, Nuembrecht, Germany) were used for blood drawing and processed within 1 h after. Turbidimetric aggregometry using a four-channel Bio/Data PAP4 aggregometer (Mölab, Langenfeld, Germany) was used to test platelet aggregation as marker for platelet reactivity as previously described [23, 25]. Platelet-rich plasma was prepared by centrifugation of citrated venous blood at 750 g for 2 min and adjusted to 275–325 × 109 thrombocytes/l by dilution with platelet-poor plasma from the same patient. To induce aggregation Adenosine diphosphate (ADP) (Sigma-Aldrich, Munich, Germany), collagen (Nycomed Pharma, Unterschleissheim, Germany), or arachidonic acid (Mölab, Hilden, Germany) were added. In Platelet-rich plasma light transmission aggregometry was performed and was determined 5 min after adding ADP at a final concentration of 5 µM or collagen 2.5 mg/L or arachidonic acid 500 mg/L. Results were expressed as percentage of maximal light transmission using platelet-poor plasma from the same patient as reference (= 100% aggregation). The used optical aggregometry assay has a coefficient of variation of 6.1% [23, 24]. Expression of P-selectin and activated GP IIb/IIIa were determined by triple color flow cytometry (FACS) after staining of the platelets with FITC-tagged PAC-1 (Becton Dickinson, Heidelberg, Germany), PE-tagged CD62P and PC7-tagged CD41 (Beckman Coulter, Krefeld, Germany) monoclonal antibodies and incubation with 20 μM ADP as described previously [25].
Lp(a) measurement
At hospital admission, levels of Lp(a) were measured as a routine laboratory parameter using an immunoturbidimetric assay (Tina-quant®, Roche, Bale, Switzerland) on a Hitachi-Modular system.
Statistical methods
For all analyses, continuous variables are presented as median ± interquartile range and discrete variables are reported as counts (percentages). We used the χ2-test to test for differences between groups of discrete variables and the Kruskal–Wallis test for non-Gaussian variables. A p value < 0.05 was regarded as significant. To analyze the impact of the Lp(a)-levels on platelet reactivity, patients were stratified into quartiles of Lp(a) levels in a similar fashion to previous studies. In addition, we investigated the influence of Lp (a) as a continuous variable using logistic regression and for correlation Spearman-Rho. Follow-up data were tested by cox regression analysis. The SPSS software package, version 25, was used for all analyses (IBM Corporation, Armonk, NY, USA). Statistical graphs were designed with GraphPad PRISM 8 (GraphPad Software, San Diego, CA, USA).
Results
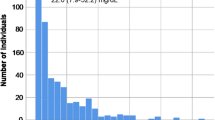
In total, 3696 patients were screened and 1912 patients with available platelet function and Lp(a) test results could be included in this analysis (Fig. 2). The median age of the patients was 66 [59–72] years and 67.9% were male. Levels of Lp(a) ranged between 0 and 332 mg/dl. There was no significant difference between quartiles of Lp(a) levels for age, left ventricular function or several cardiovascular risk factors such as arterial hypertension, diabetes mellitus, or obesity. Low-density lipoprotein cholesterol did not differ between quartiles whereas high-density lipoprotein cholesterol demonstrated significant but numerically small differences between quartiles.
The prevalence of hypercholesterolemia, a positive family history for cardiovascular disease or a previous MI, PCI or coronary artery bypass grafting increased with rising levels of Lp(a) (Table 1). A similar association was found on coronary angiography: the severity of coronary heart disease was increasing with rising levels of Lp(a). Almost 70% of patients in the highest quartile of Lp(a) levels had a coronary stenosis ≥ 75% or a stenosis ≥ 50% of the left main coronary artery. This led to an indication for PCI in more than 57% of patients in the highest quartile of Lp(a). The association of levels of Lp(a) and cardiovascular disease was also confirmed during clinical follow-up of 5.6 years. During this period, 222 (11.6%) patients died and MI occurred in 103 (5.4%) patients. Levels of Lp(a) were significantly associated with the risk of death or MI (hazard ratio 1.005; 95%-confidence interval 1.001–1.008; p = 0.01).
Platelet reactivity as tested with light transmission aggregometry did not show any differences between quartiles of Lp(a). Following stimulation with collagen or ADP, there was no significant and almost no numerical difference between quartiles for both, intrinsic and on-clopidogrel platelet reactivity (Fig. 3). Platelet reactivity following stimulation with arachidonic acid to test the effect of aspirin did also not differ between quartiles (p = 0.44). Surface protein expression of CD41, CD62P and PAC-1 following stimulation with ADP as assessed by FACS demonstrated similar results between the four quartiles of Lp(a) before as well as after loading with clopidogrel (Fig. 4). Furthermore, there was no correlation between levels of Lp(a) and results of platelet function studies (Table 2). However, platelet function correlated well with previously described predictors of platelet reactivity such as age, body mass index, diabetes, or platelet count.
Discussion
The present study investigated the clinical significance of the proposed interaction of levels of Lp(a) with platelet reactivity. The key finding of the present study is that in contrast to previous in vitro findings, the present data from a large and well powered cohort do not indicate any association of Lp(a) and in-vivo platelet function in patients on aspirin or on dual antiplatelet therapy. Results of flow cytometry analyzing surface protein expression on platelets confirmed these results. These findings, along with similar data from coagulation studies [26] showing no effect on fibrinolysis parameters with potent Lp(a) lowering treatment, suggest that the risk for coronary vascular disease associated with levels of Lp(a) may be primarily mediated by pro-atherogenic and pro-inflammatory effects via its oxidized phospholipids rather [27] than by anti-fibrinolytic or anti-platelet effects. These findings may have implications in the mechanism of any potential benefit in an ongoing trial testing the “Lp(a) hypothesis” with Lp(a) lowering by an antisense oligonucleotide (ClinicalTrials.gov Identifier: NCT04023552).
Similar results were seen in a recently published small in-vivo study that did not find any difference between intrinsic platelet reactivity in patients with levels of Lp(a) < 50 mg/dl versus ≥ 50 mg/dl [28]. These findings are in contrast to the results of in-vitro studies showing multiple different pathways of interaction between Lp(a) and platelets (Fig. 1). Experimental studies have shown that Lp(a) binds specifically and reversibly to platelets [20,21,22]. It was suggested that there might be a specific receptor for Apo(a) on platelets which modulates platelet function [21]. After activation of platelets with ADP, thrombin or arachidonic acid, the affinity of platelets to Apo(a) did not change but the binding of Lp(a) increased by 2- to 10-fold [20, 21]. However, it is still not proven so far if Apo(a) can interact with the plasminogen receptor of platelets [14] given its similar structure as compared to plasminogen. Other data suggested that Lp(a) binds to the fibrinogen (GPIIb/IIIa) receptor of platelets independently of its Apo(a) subunit [22]. However, further studies demonstrated that the IIb subunit of the fibrinogen (GPIIb/IIIa) receptor is inactivated by Apo(a), which inhibits platelet aggregation [12, 18]. Apart from different ways of binding of Lp(a) to platelets, it was shown that Lp(a) has an impact on different pathways of platelets. Cyclic AMP levels in platelets are up- or downregulated dependent on levels of Lp(a) [11]. It was demonstrated that platelet activation by ADP is inhibited by Lp(a) whereas other studies could not confirm these findings [10, 15, 18, 19]. Platelet activation promoted by the platelet-activation factor is also inhibited by Lp(a) [13, 16]. Apo(a) can lead to a decreased thromboxane A2 production in platelets and serotonin release as well as inhibition of collagen induced aggregation [10, 19]. But there are not only inhibiting effects on platelets. Other studies indicated that platelet response to the thrombin receptor-activating peptide SFLLRN is enhanced by Apo(a) [15]. It was also shown that oxidized phospholipids, which are part of Lp(a), are playing a role in atherosclerosis in particular by interacting with the CD36 receptor on platelets [17]. Binding of Apo(a) appears also to promote aggregation of platelets as demonstrated by lower doses of arachidonic acid needed to induce aggregation following binding of Lp(a) [21].
Beside inhibiting and activating effects on platelets also binding of plasminogen and tissue-type plasminogen activator to platelets is inhibited by Lp(a) and it acts as a competitive inhibitor of plasminogen activation by tissue-type plasminogen activator on the surface of platelets. Fibrinolysis might be impaired by this mechanism [20].
Lowering Lp(a) might decrease the risk for major ischemic events. Currently, only niacin, lipid apheresis, and PCSK9-inhibitors [29, 30] have shown to reduce levels of Lp(a) whereas statin therapy has demonstrated no effect or an increase in levels of Lp(a) [31]. Antisense oligonucleotide therapy to lower Lp(a) [7, 8] seems to be a promising way but is not available for clinical use so far. However, this approach has reached phase III testing and might become available in the near future. Thus, the present data might help define the potential safety of this therapeutic approach by demonstrating that the previously suggested interaction of Lp(a) and platelet function does not appear to be of importance in the target population for Lp(a) lowering.
Limitations
This analysis is retrospective with all adherent limitations. Since only patients admitted for elective coronary angiography were enrolled, the present data cannot be extrapolated to the overall population. The number of clinical endpoints within the analyzed follow-up was limited.
Conclusions
The present data do not support the hypothesis of an interaction of Lp(a) with platelet reactivity but confirms the importance of Lp(a) as risk factor for coronary events. These findings might be important to define the safety of evolving therapeutic options for lowering Lp(a).
Abbreviations
- Lp(a):
-
Lipoprotein(a)
- MI:
-
Myocardial infarction
- Apo(a):
-
Apolipoprotein(a)
- PCI:
-
Percutaneous coronary intervention
- ADP:
-
Adenosine diphosphate
References
Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG (2009) Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 301(22):2331–2339. https://doi.org/10.1001/jama.2009.801
Nordestgaard BG et al (2010) Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 31(23):2844–2853. https://doi.org/10.1093/eurheartj/ehq386
Nordestgaard BG, Langsted A (2016) Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res 57(11):1953–1975. https://doi.org/10.1194/jlr.R071233
Burgess S et al (2018) Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol 3(7):619–627. https://doi.org/10.1001/jamacardio.2018.1470
McConnell JP, Guadagno PA, Dayspring TD, Hoefner DM, Thiselton DL, Warnick GR, Harris WS (2014) Lipoprotein(a) mass: a massively misunderstood metric. J Clin Lipidol 8(6):550–553. https://doi.org/10.1016/j.jacl.2014.08.003
Varvel S, McConnell JP, Tsimikas S (2016) Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol 36(11):2239–2245. https://doi.org/10.1161/atvbaha.116.308011
Tsimikas S et al (2020) Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 382(3):244–255. https://doi.org/10.1056/NEJMoa1905239
Tsimikas S et al (2015) Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet 386(10002):1472–1483. https://doi.org/10.1016/s0140-6736(15)61252-1
Helgadottir A et al (2012) Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol 60(8):722–729. https://doi.org/10.1016/j.jacc.2012.01.078
Barre DE (1998) Lipoprotein (a) reduces platelet aggregation via apo(a)-mediated decreases in thromboxane A(2)production. Platelets 9(2):93–96. https://doi.org/10.1080/09537109876852
Barre DE (2003) Apolipoprotein (a) mediates the lipoprotein (a)-induced biphasic shift in human platelet cyclic AMP. Thromb Res 112(5–6):321–324. https://doi.org/10.1016/j.thromres.2004.01.002
Barre DE (2007) Arginyl-glycyl-aspartyl (RGD) epitope of human apolipoprotein (a) inhibits platelet aggregation by antagonizing the IIb subunit of the fibrinogen (GPIIb/IIIa) receptor. Thromb Res 119(5):601–607. https://doi.org/10.1016/j.thromres.2006.04.013
Blencowe C, Hermetter A, Kostner GM, Deigner HP (1995) Enhanced association of platelet-activating factor acetylhydrolase with lipoprotein (a) in comparison with low density lipoprotein. J Biol Chem 270(52):31151–31157. https://doi.org/10.1074/jbc.270.52.31151
Miles LA, Plow EF (1985) Binding and activation of plasminogen on the platelet surface. J Biol Chem 260(7):4303–4311
Rand ML, Sangrar W, Hancock MA, Taylor DM, Marcovina SM, Packham MA, Koschinsky ML (1998) Apolipoprotein(a) enhances platelet responses to the thrombin receptor-activating peptide SFLLRN. Arterioscler Thromb Vasc Biol 18(9):1393–1399. https://doi.org/10.1161/01.atv.18.9.1393
Tsironis LD, Mitsios JV, Milionis HJ, Elisaf M, Tselepis AD (2004) Effect of lipoprotein (a) on platelet activation induced by platelet-activating factor: role of apolipoprotein (a) and endogenous PAF-acetylhydrolase. Cardiovasc Res 63(1):130–138. https://doi.org/10.1016/j.cardiores.2004.03.005
Berliner JA, Leitinger N, Tsimikas S (2009) The role of oxidized phospholipids in atherosclerosis. J Lipid Res 50:S207-212. https://doi.org/10.1194/jlr.R800074-JLR200
Barre DE (2004) Apoprotein (A) antagonises THE GPIIB/IIIA receptor on collagen and adp-stimulated human platelets. Front Biosci 9:404–410. https://doi.org/10.2741/1194
Gries A, Gries M, Wurm H, Kenner T, Ijsseldijk M, Sixma JJ, Kostner GM (1996) Lipoprotein(a) inhibits collagen-induced aggregation of thrombocytes. Arterioscler Thromb Vasc Biol 16(5):648–655. https://doi.org/10.1161/01.atv.16.5.648
Ezratty A, Simon DI, Loscalzo J (1993) Lipoprotein(a) binds to human platelets and attenuates plasminogen binding and activation. Biochemistry 32(17):4628–4633. https://doi.org/10.1021/bi00068a021
Martinez C, Rivera J, Loyau S, Corral J, Gonzalez-Conejero R, Lozano ML, Vicente V, Angles-Cano E (2001) Binding of recombinant apolipoprotein(a) to human platelets and effect on platelet aggregation. Thromb Haemost 85(4):686–693
Malle E, Ibovnik A, Stienmetz A, Kostner GM, Sattler W (1994) Identification of glycoprotein IIb as the lipoprotein(a)-binding protein on platelets. Lipoprotein(a) binding is independent of an arginyl-glycyl-aspartate tripeptide located in apolipoprotein(a). Arterioscler Thromb 14(3):345–352. https://doi.org/10.1161/01.atv.14.3.345
Hochholzer W et al (2006) Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J Am Coll Cardiol 48(9):1742–1750. https://doi.org/10.1016/j.jacc.2006.06.065
Hochholzer W, Trenk D, Frundi D, Blanke P, Fischer B, Andris K, Bestehorn HP, Buttner HJ, Neumann FJ (2005) Time dependence of platelet inhibition after a 600-mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation 111(20):2560–2564. https://doi.org/10.1161/01.Cir.0000160869.75810.98
Hochholzer W, Trenk D, Frundi D, Neumann FJ (2007) Whole blood aggregometry for evaluation of the antiplatelet effects of clopidogrel. Thromb Res 119(3):285–291. https://doi.org/10.1016/j.thromres.2006.02.007
Boffa MB, Marar TT, Yeang C, Viney NJ, Xia S, Witztum JL, Koschinsky ML, Tsimikas S (2019) Potent reduction of plasma lipoprotein (a) with an antisense oligonucleotide in human subjects does not affect ex vivo fibrinolysis. J Lipid Res 60(12):2082–2089. https://doi.org/10.1194/jlr.P094763
Byun YS, Lee JH, Arsenault BJ, Yang X, Bao W, DeMicco D, Laskey R, Witztum JL, Tsimikas S (2015) Relationship of oxidized phospholipids on apolipoprotein B-100 to cardiovascular outcomes in patients treated with intensive versus moderate atorvastatin therapy: the TNT trial. J Am Coll Cardiol 65(13):1286–1295. https://doi.org/10.1016/j.jacc.2015.01.050
Salsoso R et al (2020) Relation of high lipoprotein (a) concentrations to platelet reactivity in individuals with and without coronary artery disease. Adv Ther 37(11):4568–4584. https://doi.org/10.1007/s12325-020-01483-y
O’Donoghue ML et al (2019) Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 139(12):1483–1492. https://doi.org/10.1161/CIRCULATIONAHA.118.037184
Bittner VA et al (2020) Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol 75(2):133–144. https://doi.org/10.1016/j.jacc.2019.10.057
Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL (2020) Statin therapy increases lipoprotein(a) levels. Eur Heart J 41(24):2275–2284. https://doi.org/10.1093/eurheartj/ehz310
Acknowledgements
Prof. Hochholzer received consultancy fees or speaker honoraria from Daiichi Sankyo, Novartis, and Bayer. Prof. Franz-Josef Neumann reports that his institution has received research grants, consultancy fees, and speaker honoraria from Daiichi Sankyo, Astra Zeneca, Sanofi-Aventis, Bayer, The Medicines Company, Bristol, Novartis, Roche, Boston Scientific, Biotronik, Medtronic, Edwards, and Ferrer. Dr. Tsimikas is a co-inventor and receives royalties from patents owned by UCSD on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins and is a co-founder and has an equity interest in Oxitope, Inc and its affiliates (“Oxitope”) as well as in Kleanthi Diagnostics, LLC (“Kleanthi”). Although these relationships have been identified for conflict of interest management based on the overall scope of the project and its potential benefit to Oxitope and Kleanthi, the research findings included in this particular publication may not necessarily relate to the interests of Oxitope and Kleanthi. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by the University Heart Center Freiburg Bad Krozingen and by a grant from Novartis.
Author information
Authors and Affiliations
Contributions
AK Writing and editing the paper, statistical analysis, figure design. TN Writing and editing the paper. KF Writing and editing the paper. CV Writing and editing the paper. GL Writing and editing the paper, designed Fig. 1. ST Writing and editing the paper. FJN Writing and editing the paper. WH designed and supervised the study and designed the database. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All other authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kille, A., Nührenberg, T., Franke, K. et al. Association of lipoprotein(a) with intrinsic and on-clopidogrel platelet reactivity. J Thromb Thrombolysis 53, 1–9 (2022). https://doi.org/10.1007/s11239-021-02515-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02515-2