Abstract
Background
The oral anticoagulant dabigatran offers an effective alternative to vitamin K antagonists (VKAs) for stroke prevention in atrial fibrillation (AF), yet patient preference data are limited. The prospective observational RE-SONANCE study demonstrated that patients with AF, newly initiated on dabigatran, or switching to dabigatran from long-term VKA therapy, reported improved treatment convenience and satisfaction compared with VKA therapy. This pre-specified sub-study aimed to assess the impact of country and age on patients’ perceptions of dabigatran or VKA therapy in AF.
Methods
RE-SONANCE was an observational, prospective, multi-national study (NCT02684981) that assessed treatment satisfaction and convenience in patients switching from VKAs to dabigatran (Cohort A), or newly diagnosed with AF receiving dabigatran or VKAs (Cohort B), using the PACT-Q questionnaire. Pre-specified exploratory outcomes: variation in PACT-Q2 scores by country and age (< 65, 65 to < 75, ≥ 75 years) (both cohorts); variation in PACT-Q1 responses at baseline by country and age (Cohort B).
Results
Patients from 12 countries (Europe/Israel) were enrolled in Cohort A (n = 4103) or B (n = 5369). In Cohort A, mean (standard deviation) PACT-Q2 score increase was highest in Romania (convenience: 29.6 [23.6]) and Hungary (satisfaction: 26.0 [21.4]) (p < 0.001). In Cohort B, mean (standard error) increase in PACT-Q2 scores between dabigatran and VKAs was highest in Romania (visit 3: 29.0 [1.3]; 24.5 [0.9], p < 0.001). Mean PACT-Q2 score increase by age (all p < 0.001) was similar across ages. PACT-Q1 responses revealed lowest expectations of treatment success in Romania and greatest concerns about payment in Estonia, Latvia, and Romania, but were similar across ages.
Conclusions
Treatment satisfaction and convenience tended to favor dabigatran over VKAs. Regional differences in treatment expectations exist across Europe.
Trial and clinical registry
Trial registration number: ClinicalTrials.gov NCT02684981.
Trial registration date: February 18, 2016.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
This was an exploratory analysis of the RE-SONANCE study.
-
AF patients from Europe and Israel prescribed dabigatran or VKA were included.
-
Perceptions of anticoagulant treatment were assessed by country and age via PACT-Q.
-
Treatment satisfaction and convenience tended to favor dabigatran over VKAs.
-
Regional differences in treatment expectations in AF exist across Europe.
Introduction
Current treatment guidelines recommend oral anticoagulant (OAC) therapy for patients with atrial fibrillation (AF) at risk of thromboembolic events or stroke [1, 2]. For many years, vitamin K antagonists (VKAs), such as warfarin, have been the only available OAC therapy for patients with AF who are at risk of stroke. However, their narrow therapeutic window, need for dose adjustment and monitoring, risk of intracranial hemorrhage, and multiple interactions with other drugs and food make VKAs difficult to manage in routine clinical practice, resulting in underuse and suboptimal adherence [3,4,5]. A number of non-VKA oral anticoagulants (NOACs) have since been introduced as an alternative to VKA therapy.
NOAC treatment, including the direct thrombin inhibitor dabigatran, in patients with AF who are indicated for anticoagulation treatment has shown to be just as effective as VKA treatment in reducing the risk of stroke (dabigatran 150 mg twice daily is associated with lower rates of stroke and systemic embolism compared with warfarin) [6]. Importantly, NOACs are not associated with the same pharmacological issues as VKAs that affect adherence.
Current guidelines for stroke prevention recommend the involvement of patients in treatment decisions [1, 2, 7], and patient preference may be key in adherence to long-term anticoagulant therapy. Previous studies have reported mixed outcomes regarding quality of life (QoL) and patient preferences for NOAC and VKA therapy for stroke prevention in AF [8,9,10,11]. Nevertheless, there remain limited data on patient preference regarding NOAC and VKA therapy, particularly in terms of country- or age-specific preferences.
The prospective observational RE-SONANCE study, conducted using the validated Perception of Anticoagulant Treatment Questionnaire (PACT-Q), demonstrated that patients with non-valvular AF, newly initiated on dabigatran, or switching to dabigatran from long-term VKA therapy, reported improved treatment convenience and satisfaction compared with VKA therapy [12]. In this pre-specified, exploratory analysis from the RE-SONANCE study, variations in treatment convenience and satisfaction (PACT-Q2) scores between countries and age groups were examined. Variations in treatment expectation (PACT-Q1) scores at baseline between countries and age groups were also explored.
Methods
Patients and study design
Methods for the RE-SONANCE study have been described previously [12]. In brief, RE-SONANCE was an observational, multi-national study (NCT02684981) conducted in Austria, Bulgaria, the Czech Republic, Estonia, Hungary, Israel, Latvia, Poland, Romania, Russia, Serbia, and Slovenia. The study included physiologists who worked at sites that reflected clinical practice in their country and regularly prescribed dabigatran and VKA for stroke prevention in patients with AF. Patients enrolled were at least 18 years old with non-valvular AF and an indication for anticoagulation therapy for stroke prevention, and not currently participating in any clinical trial or registry. Patients were assigned to 1 of 2 cohorts: Cohort A, which comprised patients with non-valvular AF who were switched from a VKA to dabigatran based on the product label and at the physician’s discretion (≥ 3 months’ continuous VKA treatment for stroke prevention prior to baseline); or Cohort B, which comprised patients newly diagnosed with AF initiated on either dabigatran or VKA (without use of any OAC within 1 year before enrollment). This study was carried out in accordance with the Declaration of Helsinki, International Conference of Harmonisation Tripartite Guideline, Good Clinical Practice, Guidelines for Good Epidemiological Practice and Good Pharmacoepidemiology Practice. The study was initiated in centers once approved by the respective Institutional Review Board/Independent Ethics Committee and competent authority, according to national and international regulations.
Assessments and outcomes
Patient characteristics and treatment data were collected during routine clinic visits (V) over an observation period of approximately 6 months, at three recommended time points: V1 (baseline; switch from VKA to dabigatran, or initiation of dabigatran/VKA), V2 (30–45 days after baseline), and V3 (150–210 days after baseline). However, if visits occurred outside these time periods, data were collected and assigned to visits based on: V2, 7–124 days after baseline; V3, 125–356 days after baseline. Patients completed the self-administered PACT-Q face-to-face in the clinic [13, 14].
PACT-Q1 assessed patient expectations of anticoagulation therapy, and was administered at V1 for Cohort B only. PACT-Q2 assessed convenience, burden of disease and treatment, and anticoagulant treatment satisfaction; it was administered at V1 (Cohort A only), V2, and V3 (Cohorts A and B). For more information on PACT-Q, refer to Vinereanu et al. [12].
Pre-specified exploratory outcomes included the variation of PACT-Q2 scores across different countries, and in different age groups (< 65, 65 to < 75, and ≥ 75 years) (Cohorts A and B); and variation in PACT-Q1 scores at V1 in different age groups, and geographical variation in treatment perception (Cohort B).
Statistical methods
The enrolled population comprised all patients who fulfilled the eligibility criteria. The main analysis set (MAS) comprised all eligible patients with known treatment. The propensity score matched set in Cohort B comprised all patients matched with a 1:n ratio (VKA:dabigatran) based on propensity scores calculated using a logistic regression model. Summary statistics were prepared for demographic and baseline characteristics, healthcare system characteristics, and physician-rated risk scores, based on the MAS [12].
Assessment of PACT-Q2 scores
For Cohort A, mean differences in PACT-Q2 scores between visits were assessed using paired t-tests or signed-rank Wilcoxon test. For Cohort B, mean differences in PACT-Q2 scores between treatment subgroups were assessed using propensity score matched analysis. The baseline variables used in the propensity score matched analysis included sex (male/female); age (< 65, ≥ 65 to < 75, ≥ 75 years); status of reimbursement for anticoagulation therapy (reimbursed, partially reimbursed, private pay, other); specialty of the treating physician (cardiologist, internist, neurologist, general practitioner, other); Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Pre-disposition, Labile international normalized ratio, Elderly, Drugs/Alcohol Concomitantly (HAS-BLED) score (low risk [< 3], high risk [≥ 3]); CHA2DS2-VASc (Congestive heart failure, Hypertension, Age (≥ 75), Diabetes mellitus, Stroke/transient ischemic attack, Vascular disease, Age 65–75, Sex category) score for embolic risk assessment (considered low or intermediate risk if < 2, and high risk if ≥ 2); number of concomitant medications (0, 1–3, ≥ 4); type of concomitant medication (prescription or no prescription of anti-arrhythmics, antiplatelets, or non-steroidal anti-inflammatory drugs); number of concomitant therapies (0, ≥ 1); presence of comorbidities (presence or absence of malignancy, or gastroesophageal reflux disease or gastroduodenal ulcer disease). Due to the variable size of the matched sets, the analysis of PACT-Q2 scores was also based on the random intercept model, where a variance in the unequal groups (1:n matching) was compared and one group contained “repeated” observations. For sub-group analysis by country, a minimum sample size of 250 patients for Cohort A and 260 for Cohort B was required. For other sub-groups, a minimum sample size of 100 patients for Cohort A and 200 for Cohort B was required.
Assessment of PACT-Q1 scores
For Cohort B, PACT-Q1 scores at baseline were summarized descriptively for all patients and between treatment sub-groups.
Results
Patients
A total of 9472 patients with AF were enrolled (enrolled population) from 698 sites in 12 countries (Europe and Israel) to Cohort A (n = 4103) or Cohort B (n = 5369). The MAS comprised 4100 patients in Cohort A and 5365 in Cohort B (dabigatran, n = 3179; VKA, n = 2186) [12]. Baseline characteristics are summarized in Table 1. As noted previously, the mean (standard deviation) age of patients at baseline in Cohort A was 70.5 (9.6) years and 68.6 (9.9) years in Cohort B. In general, age groups were evenly distributed between cohorts, with fewer patients aged < 65 years in Cohort A.
A high risk of systemic embolism (CHA2DS2-VASc score ≥ 2) and a high risk of bleeding complications (HAS-BLED score ≥ 3) were observed in Cohort A (88.3 and 59.2% of patients, respectively) and Cohort B (dabigatran, 87.8 and 29.1% of patients, respectively; VKA, 91.4 and 31.3% of patients, respectively). Most patients had comorbidities and were receiving concomitant medications. Most patients receiving dabigatran in both cohorts received 150 mg twice daily (Table 1). In Cohort A, the mean duration of previous VKA therapy was 34 months (median 19 months).
Treatment convenience and satisfaction (Cohorts A and B)
European cohorts overall
Among the patients switching from a VKA to dabigatran (Cohort A) in the overall analysis set, PACT-Q2 improved significantly for treatment convenience and treatment satisfaction. Mean change for treatment convenience from V1 to V3 was 24.54 (standard deviation [SD] 22.85) and mean change for treatment satisfaction was 21.04 (SD 20.24) (p < 0.001). Among the newly initiated patients in Cohort B, PACT-Q2 scores also showed significant improvement for dabigatran versus a VKA (p < 0.001). Mean difference in PACT-Q2 scores between dabigatran and VKA at V3 for treatment convenience was 23.34 (standard error [SE] 0.51), and for treatment satisfaction was 19.01 (SE 0.41) [12].
By country
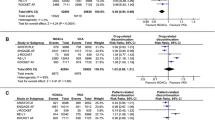
Of patients switching from a VKA to dabigatran (Cohort A), a statistically significant mean increase in convenience and treatment satisfaction scores (PACT-Q2 items) between visits was observed only in the Czech Republic, Hungary, Poland, Romania, and Russia (all p < 0.001). Changes from V1 to V3 are shown in Fig. 1a. Other countries could not be assessed due to low sample sizes: changes in Austria, Bulgaria, Estonia, Israel, Latvia, Serbia, and Slovenia were statistically inconclusive.
PACT-Q2 score changes in treatment convenience and satisfaction by country (Cohort A, main analysis set; Cohort B, propensity score matched set). a Cohort A: patients switched from a VKA to dabigatran; change from V1 to V3. b Cohort B: patients newly initiated on dabigatran or a VKA; difference between dabigatran and VKA at V3. Data for the Czech Republic and Hungary are not presented in Fig. 1b because patient numbers meant that changes were statistically inconclusive. All changes were p < 0.001 and in favor of the dabigatran sub-group. PACT-Q, Perception of Anticoagulant Treatment Questionnaire; SD, standard deviation; SE, standard error; V1, baseline; V2, initiation period; V3, continuation period; VKA, vitamin K antagonist
Within Cohort B (newly initiated patients), significant changes in convenience and satisfaction between VKA and dabigatran sub-groups were observed at V2 and V3 for Poland, Romania, and Russia (p < 0.001). These changes favored the dabigatran sub-group and tended to be higher at V3 versus V2. Differences between dabigatran and VKA at V3 are shown in Fig. 1b. In some countries, the changes in convenience and satisfaction could not be assessed in Cohort B (Czech Republic and Hungary) or both cohorts (Austria, Bulgaria, Estonia, Israel, Latvia, Serbia, and Slovenia) due to low sample sizes.
By age group
Treatment convenience and satisfaction (PACT-Q2 items) among different age groups (< 65, 65 to < 75, ≥ 75 years) in Cohorts A and B are summarized in Fig. 2a and b. Changes in convenience and satisfaction scores between visits (Cohort A) and between dabigatran and VKA sub-groups at V2 and V3 (Cohort B) were generally consistent between age groups. Changes between visits in convenience and satisfaction within all three age groups in both cohorts were statistically significant (p < 0.001), and favored the dabigatran sub-group.
PACT-Q2 score changes in treatment convenience and satisfaction by age group (Cohort A, analysis set; Cohort B, propensity score matched set). a Cohort A: patients switched from a VKA to dabigatran; change from V1 to V3. b Cohort B: patients newly initiated on dabigatran or a VKA; difference between dabigatran and VKA at V3. All changes were p < 0.001 and in favor of the dabigatran sub-group. PACT-Q, Perception of Anticoagulant Treatment Questionnaire; SD, standard deviation; SE, standard error; V1, baseline; V2, initiation period; V3, continuation period; VKA, vitamin K antagonist
Treatment expectations at baseline (Cohort B)
By country
Table 2 summarizes treatment perceptions (PACT-Q1 items) at baseline in patients newly initiated on a VKA or dabigatran (Cohort B) across countries. Low sample sizes meant that some data could not be interpreted (Czech Republic, Hungary, and Slovenia, where N < 30) or should be interpreted with caution (Bulgaria, Estonia, Israel, and Latvia, where N = 50–100). Overall, most patients were confident that their anticoagulant therapy would prevent blood clots. Most patients in Austria reported this to be either “extremely” (39.8%) or “a lot” (39.5%), while most patients in other countries reported either “moderately” or “a lot.” The expectation that the anticoagulant therapy would provide symptom relief ranged from “a little” to “a lot” for all. Patients in Estonia and Israel had the highest expectations (34.3 and 35.3% reported “a lot,” respectively), while a large proportion of Romanian patients had no expectations (21.5% reported “not at all”). Expectations regarding side effects differed between countries, but with the exception of Austria, Russia, Romania, and Estonia, most patients reported moderate expectations that their anticoagulant therapy would cause side effects such as minor bruises or bleeding. Most patients in all countries considered it important to have an anticoagulant treatment that was easy to take (reporting “a lot” or “extremely”), yet a large proportion of patients in Bulgaria (21.6%), Estonia (17.6%), Israel (29.4%), and Russia (18.3%) found this of only moderate importance. A large proportion of patients in Bulgaria (47.1%) and Israel (41.2%) had moderate concerns about making mistakes when taking their anticoagulant therapy, while Austrian patients reported the least concern (44.2% responded “not at all”). In most countries, a large proportion of patients believed it was important to take care of the anticoagulant therapy themselves (20.8–71.4% responded “a lot”). Notably, a large proportion of patients in Austria (54.4%) and Serbia (42.4%) believed this to be extremely important. Concern about payment for their anticoagulant therapy varied from “moderate” to “a lot” in most countries, with many patients in Estonia (29.4%), Latvia (23.3%), and Romania (26.0%) expressing extreme concern. This contrasts with Austrian patients, where a large proportion (40.1%) had no concern about payment at all. Differences were also observed between dabigatran and VKA sub-groups (see Table 1 of the supplementary material).
By age group
The variations of treatment expectations in different age groups measured by PACT-Q1 for Cohort B are summarized in Table 3. Overall, the distribution of answers to PACT-Q1 treatment expectation questions was similar between the three different age groups. Large proportions of patients in all age groups were confident that their anticoagulant treatment would prevent blood clots (39.4–50.0% answered “a lot”), found it important to have an anticoagulant treatment that was easy to take (52.6–52.9% answered “a lot”), and found it important for them to take care of their anticoagulant treatment by themselves (48.9–51.2% answered “a lot”). When asked about their confidence that the anticoagulant treatment would prevent blood clotting, a slightly higher proportion of patients in the < 65 years group (50.0%) answered “a lot” compared with the ≥ 75 years group (39.4%). Similar numbers across the three age groups expected their anticoagulant treatment to provide symptom relief (27.7–28.8% answered “a lot”, and 29.2–30.7% answered “moderately”), and expected their anticoagulant treatment to cause side effects (31.9–35.9% answered “moderately”, and 34.7–39.1% answered “a little”). Patient concerns about making mistakes when taking their medication ranged across all answers in all three age groups. Most patients answered “moderately” (26.6–27.6%) and “a lot” (27.6–30.9%) when asked about their concerns regarding payment for their treatment.
When comparing the dabigatran and VKA sub-groups of Cohort B according to age group (see Tables 2, 3 and 4 of the supplementary material), a higher proportion of patients aged 65 to < 75 years in the dabigatran sub-group versus the VKA sub-group were extremely confident that their anticoagulant treatment would prevent blood clots (16.7% vs. 9.7% answered “extremely”, respectively) and for patients aged ≥ 75 years, 18.2% versus 11.1% answered “extremely”, respectively. Among patients aged < 65 years and 65 to < 75 years, more patients in the VKA sub-group versus the dabigatran sub-group were concerned about making mistakes when taking their medication (< 65 years, 31.8% vs. 23.0% answered “moderately”, respectively; but those aged 65 to < 75 years, 30.6% vs. 24.4% answered “a lot”, respectively).
Discussion
This exploratory analysis was conducted as part of the observational RE-SONANCE study to investigate patient perceptions of anticoagulation treatment while receiving dabigatran or VKA for stroke prevention in AF in Europe (11 countries) and Israel. Perceptions were assessed by country and by age group. Assessment of treatment convenience and satisfaction (PACT-Q2) scores in different countries in Cohort A (Czech Republic, Hungary, Poland, Romania, and Russia) confirmed improved treatment satisfaction and convenience in favor of dabigatran compared with VKAs in patients who switched from VKA to dabigatran. Similarly, differences in PACT-Q2 scores between dabigatran and VKA sub-groups in Cohort B (Poland, Romania, and Russia) confirmed improved treatment satisfaction and convenience in favor of dabigatran compared with VKAs in patients newly initiated on either anticoagulant. Data from other countries could not be assessed due to low patient numbers. Changes in treatment convenience and satisfaction (PACT-Q2) scores were generally consistent across age groups and favored the dabigatran sub-group.
Assessment of treatment expectation (PACT-Q1) responses at baseline in Cohort B showed differences between countries. This reflects different perceptions of oral anticoagulation in patients with AF between different countries. This issue was also apparent in the IMPACT-AF trial [15]. Patients in Austria tended to be the most confident that their anticoagulant therapy would prevent blood clots. Patients in Estonia had the highest expectations regarding symptom relief, while a large proportion of patients in Romania had no expectations. Patients in Austria reported the least concern about making mistakes when taking their medication and, together with patients in Serbia, believed it was extremely important to take care of the anticoagulant therapy themselves. Extreme concerns regarding payment were expressed by patients in Estonia, Latvia, and Romania, while many Austrian patients had no concerns. The regional differences in treatment expectations across Europe highlighted in this analysis suggest the need for improvements in patient education regarding anticoagulation therapy. Assessment of treatment expectation (PACT-Q1) responses at baseline between different age groups in Cohort B revealed that overall, patients aged < 65 years tended to be more confident that their medication would prevent blood clotting compared with those aged ≥ 75 years. In the dabigatran sub-group versus the VKA sub-group, patients aged 65 to < 75 years and ≥ 75 years also tended to be more confident that their treatment would prevent blood clots.
This exploratory analysis of RE-SONANCE study data is subject to the limitations inherent to observational studies and exploratory analyses. The results were also influenced by low sample sizes. When assessing PACT-Q2 scores by country, changes in Austria, Bulgaria, Estonia, Israel, Latvia, Serbia, and Slovenia were statistically inconclusive. When assessing changes in PACT-Q1 scores in Cohort B, data could not be interpreted (Czech Republic, Hungary, and Slovenia) or should be interpreted with caution (Bulgaria, Estonia, Israel, and Latvia). Propensity score matching was conducted to control for channeling bias in Cohort B, but the model considered a limited number of comorbidities; those not included have the potential to cause residual confounding. Other potential sources of bias include reimbursement for anticoagulation therapy; patients may have been unwilling to express their true opinions in PACT-Q, or had difficulty in understanding the questions; those switching to dabigatran may have subconsciously considered the new therapy better than their previous therapy, particularly as the clinical reasons for switching from a VKA to dabigatran were not collected.
These limitations notwithstanding, data from this exploratory analysis support and expand upon published results from the RE-SONANCE study and resonate with other studies. When compared with the overall RE-SONANCE population, improvements in both treatment convenience and satisfaction (PACT-Q2) scores tended to be numerically higher in Hungary (Cohort A), Poland, and Romania (Cohorts A and B) [12]. Findings among the different age groups (< 65, 65 to < 75, and ≥ 75 years), however, were generally consistent with the overall population. Other than RE-SONANCE, few other studies have assessed patients’ perspectives of anticoagulant therapy in AF and, in particular, how these vary between countries and age groups [12]. A cross-sectional study conducted in France described real-world experience of direct OACs or VKAs for AF, and found that although patients in both groups had comparable QoL and adherence, those receiving a NOAC reported significantly greater treatment satisfaction (using PACT-Q2) [11]. QoL data from the phase III RE-LY® trial, which included patients from 44 countries, revealed comparable EuroQol 5 dimensions (EQ-5D) utility and visual analog scores in the dabigatran and warfarin groups after 12 months of stable treatment [8,6]. The Prevention of Thromboembolic Events—European Registry in Atrial Fibrillation (PREFER in AF) Registry assessed QoL (EQ-5D-5 L) and satisfaction (PACT-Q2) in patients on stable treatment with a VKA, or those recently switched from a VKA to a NOAC [10]. Patients who had switched to NOACs from VKAs tended to be at lower risk than non-switchers and dissatisfied with VKA treatment. The need for increased patient awareness regarding OACs has been recognized previously [16, 17]. Therefore, as current guidelines for stroke prevention recommend patient involvement in treatment decisions, it will be important to increase patient confidence in dabigatran.
Conclusions
This pre-specified, exploratory analysis from RE-SONANCE provides further confirmation of a preference toward switching to dabigatran from VKAs, or initiating dabigatran versus a VKA for stroke prevention in newly diagnosed patients with AF. This analysis highlights regional differences in treatment expectations across Europe.
Data availability
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfill their role and obligations as authors under the ICMJE criteria. Furthermore, clinical study documents (e.g. study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the BI Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/. Prior to providing access, documents will be examined, and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants. Clinical Study Reports and Related Clinical Documents can also be requested via the link https://trials.boehringer-ingelheim.com/. All requests will be governed by a Document Sharing Agreement. Bona fide, qualified scientific and medical researchers may request access to de-identified, analysable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request. Researchers should use the https://trials.boehringer-ingelheim.com/ link to request access to study data.
References
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, ESC Scientific Document Group (2016) 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37(38):2893–2962
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW (2019) 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation 140(2):e125–e151
Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S (2007) Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 115(21):2689–2696
Birman-Deych E, Radford MJ, Nilasena DS, Gage BF (2006) Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke 37(4):1070–1074
Jones M, McEwan P, Morgan CL, Peters JR, Goodfellow J, Currie CJ (2005) Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. Heart 91(4):472–477
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee, Investigators (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361(12):1139–1151
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbuchel H, ESC Scientific Document Group (2018) The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 39(16):1330–1393
Monz BU, Connolly SJ, Korhonen M, Noack H, Pooley J (2013) Assessing the impact of dabigatran and warfarin on health-related quality of life: results from an RE-LY sub-study. Int J Cardiol 168(3):2540–2547
Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R (2017) Patient preferences for oral anticoagulation therapy in atrial fibrillation: a systematic literature review. Patient 10(1):17–37
De Caterina R, Bruggenjurgen B, Darius H, Kohler S, Lucerna M, Pecen L, Renda G, Schilling RJ, Schliephacke T, Zamorano JL, Le Heuzey JY, Kirchhof P (2018) Quality of life and patient satisfaction in patients with atrial fibrillation on stable vitamin K antagonist treatment or switched to a non-vitamin K antagonist oral anticoagulant during a 1-year follow-up: a PREFER in AF Registry substudy. Arch Cardiovasc Dis 111(2):74–84
Benzimra M, Bonnamour B, Duracinsky M, Lalanne C, Aubert JP, Chassany O, Aubin-Auger I, Mahe I (2018) Real-life experience of quality of life, treatment satisfaction, and adherence in patients receiving oral anticoagulants for atrial fibrillation. Patient Prefer Adherence 12:79–87
Vinereanu D, Napalkov D, Bergler-Klein J, Benczur B, Ciernik M, Gotcheva N, Medvedchikov A, Poder P, Simic D, Skride A, Tang W, Trusz-Gluza M, Vesely J, Vishnepolsky T, Vrabec M (2020) Patient perception of anticoagulant treatment for stroke prevention (RE-SONANCE study). Open Heart 7(1):e001202
Prins MH, Guillemin I, Gilet H, Gabriel S, Essers B, Raskob G, Kahn SR (2009) Scoring and psychometric validation of the Perception of Anticoagulant Treatment Questionnaire (PACT-Q). Health Qual Life Outcomes 7:30
Prins MH, Marrel A, Carita P, Anderson D, Bousser MG, Crijns H, Consoli S, Arnould B (2009) Multinational development of a questionnaire assessing patient satisfaction with anticoagulant treatment: the ‘Perception of Anticoagulant Treatment Questionnaire’ (PACT-Q). Health Qual Life Outcomes 7:9
Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al-Khalidi HR, He W, Xian Y, Ciobanu AO, Kamath DY, Fox KA, Rao MP, Pokorney SD, Berwanger O, Tajer C, de Barros ESPGM, Roettig ML, Huo Y, Granger CB, IMPACT-AF investigators (2017) A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet 390(10104):1737–1746
Amara W, Larsen TB, Sciaraffia E, Hernandez Madrid A, Chen J, Estner H, Todd D, Bongiorni MG, Potpara TS, Dagres N, Sagnol P, Blomstrom-Lundqvist C (2016) Patients’ attitude and knowledge about oral anticoagulation therapy: results of a self-assessment survey in patients with atrial fibrillation conducted by the European Heart Rhythm Association. Europace 18(1):151–155
Cimminiello C, Hatala R, Pakarinen S, Friz HP, Fitzmaurice D, Hobbs FR (2018) Non-vitamin k antagonist oral anticoagulants in a European primary care physician survey. BJGP Open 2(3):bjgpopen18X101602
Acknowledgements
The authors thank all the patients who participated in this study. Medical writing support was provided by Parexel, with funding from Boehringer Ingelheim GmbH.
Funding
This study was funded by Boehringer Ingelheim GmbH. Boehringer Ingelheim GmbH sponsored the study, contributed to the study design, and was involved in data collection, drafting and reviewing of the manuscript, and provided final approval of the manuscript, as listed in the Authors’ Contributions.
Author information
Authors and Affiliations
Contributions
DV and AM: contributed to the study design and were involved in data collection and interpretation; DN, JB-K, BB, NG, PP, DS, AS and JV: were involved in data collection and interpretation; MC: was involved in data interpretation; WT: was involved in data analysis; MT-G: was involved in data collection (national study coordinator). All authors: contributed to data collection and interpretation, are accountable for the work, contributed to the drafting and reviewing of the manuscript, and provided final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dragos Vinereanu received grants and personal fees from Boehringer Ingelheim during the conduct of the study; grants and personal fees from Bristol-Myers Squibb, Pfizer, Bayer, Johnson & Johnson, and Daiichi Sankyo outside the submitted work. Dmitry Napalkov received research grants and has participated in scientific advisory boards for Boehringer Ingelheim and has received speaker’s honoraria from Bayer, Boehringer Ingelheim, Pfizer, and Takeda. Bela Benczur received speaker’s/consultancy fees from Bayer, Berlin-Chemie/Menarini, Boehringer Ingelheim, Krka Pharma, Novartis, Pfizer, Sandoz, and Sanofi. Martin Ciernik, Alexey Medvedchikov, and Wenbo Tang are employees of Boehringer Ingelheim. Pentti Põder received educational grants from Boehringer Ingelheim. Maria Trusz-Gluza received personal fees from Boehringer Ingelheim (null during the conduct of the study), personal fees and non-financial support from Boehringer Ingelheim, and personal fees from Bayer outside the submitted work. Jiří Vesely received personal fees and non-financial support from Bayer, Boehringer Ingelheim, Pfizer, MSD, and PRO.MED.CS outside the submitted work. All other authors report no conflicts of interest.
Ethical approval
This study was carried out in accordance with the Declaration of Helsinki, International Conference of Harmonisation Tripartite Guideline, Good Clinical Practice, Guidelines for Good Epidemiological Practice and Good Pharmacoepidemiology Practice. The study was initiated in centers once approved by the respective Institutional Review Board/Independent Ethics Committee and competent authority, according to national and international regulations.
Informed consent
Written informed consent was obtained from eligible patients prior to participation.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vinereanu, D., Napalkov, D., Bergler-Klein, J. et al. Patient perceptions of anticoagulant treatment with dabigatran or a vitamin K antagonist for stroke prevention in atrial fibrillation according to region and age: an exploratory analysis from the RE-SONANCE study. J Thromb Thrombolysis 52, 1195–1206 (2021). https://doi.org/10.1007/s11239-021-02450-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02450-2