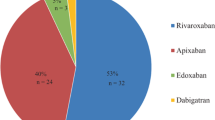
Abstract
In randomized clinical trials (RCTs) of nonvitamin K antagonist oral anticoagulants (NOACs) for acute venous thromboembolism (VTE), ~ 12–13% of patients were elderly and ~ 26% had mild-to-moderate renal impairment. Observational studies are not restricted by the selection and treatment criteria of RCTs. In this ancillary analysis of the RE-COVERY DVT/PE global observational study, we aimed to describe patient characteristics, comorbidities, and anticoagulant therapy for subgroups of age (< or ≥ 75 years) and renal impairment (creatinine clearance [CrCl; estimated with Cockcroft–Gault formula] < 30 [severe], 30 to < 50 [moderate], 50 to < 80 [mild], ≥ 80 [normal] mL/min). Of 6095 eligible patients, 25.3% were aged ≥ 75 years; 38.2% (1605/4203 with CrCl values) had mild-to-moderate renal impairment. Comorbidities were more common in older patients (73.9% aged ≥ 75 vs. 58.1% < 75 years) and in those with mild or moderate versus no renal impairment (75.9%, 80.9%, and 59.3%, respectively). At hospital discharge or 14 days after diagnosis (whichever was later), most patients (53.7% and 55.1%, respectively) in both age groups received NOACs; 20.8% and 23.4%, respectively, received vitamin K antagonists, 19.0% and 21.8% parenteral therapy, 2.3% and 3.8% other anticoagulant treatments. Use of NOACs decreased with worsening renal impairment (none 58.5%, moderate 49.6%, severe 25.7%) and, in younger versus older patients with moderate renal impairment (33.1% vs. 56.1%). In routine practice, there are more elderly and renally impaired patients with VTE than represented in RCTs. Decreasing renal function, but not older age, was associated with less NOAC use. Clinical Trial Registration: http://www.clinicaltrials.gov. Unique identifier: NCT02596230.
Graphic abstract
Decreasing renal function, particularly in the subgroup with CrCl < 30 mL/min, but not older age, was associated with less use of nonvitamin K antagonist oral anticoagulants (NOACs). Nevertheless, more than half of the older patients with moderate renal impairment received a NOAC as their oral anticoagulant.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
RE-COVERY DVT/PE was a study of acute venous thromboembolism treatment in routine clinical practice.
-
Compared with randomized clinical trials, more patients were elderly and renally impaired.
-
Over half of the patients received nonvitamin K antagonist oral anticoagulants (NOACs), suggesting an increased confidence with these drugs in elderly populations.
-
Decreasing renal function, but not older age, was associated with less NOAC use.
-
The observed approach is supported by the results of randomized clinical trials that showed a better safety profile of NOACs compared with VKAs, particularly in elderly patients.
Introduction
The risk of venous thromboembolic events (VTEs), comprising deep vein thrombosis (DVT) and/or pulmonary embolism (PE), increases with age [1] and chronic kidney disease [2]. Sparse data are available to help guide clinicians in choosing the optimal anticoagulation regimens for elderly or renally impaired patients, who are often excluded from randomized clinical trials (RCTs).
Current guidelines suggest the use of nonvitamin K antagonist oral anticoagulants (NOACs) over vitamin K antagonists (VKAs) for the treatment of acute VTE [3], based on the similar efficacy and improved safety of NOACs versus VKAs in the RCT patient populations [4,5,6,7]. However, the guidelines [3] do not provide specific recommendations for treating elderly and/or renally impaired patients, but the product labels for NOACs provide specific recommendations for dose adjustment in these patients [8,9,10].
The RE-COVERY DVT/PE study has two objectives. In the first phase, it aims to characterize patients with VTE, the location of VTE events, and treatment patterns at initial presentation [11]. In the second phase, it evaluates outcomes in patients treated with dabigatran or VKA in routine clinical practice [12]. We previously described baseline characteristics of the overall cohort of patients in the first phase, regional variations in treatment choices, and the influence of baseline clinical features such as cancer on the choice of anticoagulants [11]. Overall, 77% of patients received oral anticoagulants (54% NOACs and 23% VKAs), and 20% received parenteral anticoagulation only. NOACs comprised about 60% of anticoagulant treatment in Europe and Asia but less than a third in Latin America and the Middle East. The proportion of patients treated with NOACs was lower among those with cancer, chronic renal disease, heart failure, or stroke than in those without these comorbidities. Irrespective of the index event (DVT, PE, DVT, and PE), NOACs were the most common choice of anticoagulants. However, analysis of standardized differences suggested there was lower use of NOACs in patients with co-existing DVT and PE (49.5%) compared with DVT alone (54.9%). The pattern of NOAC use did not vary notably according to location of DVT (distal, proximal, upper limb, or other).
In the current report using demographic data from the first phase of the study, we evaluate the profile of patients treated for acute VTE in routine clinical practice according to their age and renal function and to compare it, where feasible, with the profiles of patients in randomized clinical trials of dabigatran. Furthermore, we also aimed to investigate the anticoagulant treatment strategies used in the different age and renal function subgroups.
Methods
Study design
The rationale and design of the large, multicenter, international RE-COVERY DVT/PE observational study have been described previously [12]. In the first phase, patients with acute VTE were characterized according to baseline features and initial treatment. Patients could be entered into the study up to 6 months following the acute event. Investigators were encouraged to include consecutive patients with acute VTE, irrespective of initial treatment. In the second phase, safety and effectiveness outcomes over a follow-up period of 1 year were compared for dabigatran and VKA.
The study was carried out in compliance with the protocol and the principles laid down in the Declaration of Helsinki. In addition, the applicable sections of the guidelines for Good Clinical Practice, Good Epidemiological Practice, and Good Pharmacoepidemiology Practices, and local regulations were followed. Patients (or their legal representative) provided written informed consent before study entrance, in accordance with local regulations. No study procedures or data recording were performed unless a patient had consented to participate in the study or a waiver had been obtained in accordance with local regulations.
Eligibility criteria
Eligible patients included those aged ≥ 18 years and diagnosed with acute proximal or distal DVT and/or PE. If anticoagulation was required for any condition other than VTE, or if patients were participating in another clinical trial for VTE, they were excluded.
Data collection
Following a diagnosis of VTE, patient characteristics and anticoagulant treatment administered at baseline were recorded. As treatment with some oral anticoagulants may be preceded by parenteral anticoagulation with heparin or fondaparinux, anticoagulant therapy was recorded again at hospital discharge or at 14 days after the diagnosis, whichever was later. Sites recorded all clinical data and site/investigator characteristics via a secure, web-based, electronic data capture system. Potential selection bias was minimized by using consecutive, unselected enrollment (regardless of a patient’s treatment or management).
Statistical analysis
Approximately 6000 patients were planned to be enrolled in phase 1 of the study. The sample size was not based on formal sample size calculations, as no a priori hypothesis testing was involved. Based on a range of prevalence rates of events or patient attributes and the width of the associated 95% confidence intervals, a sample size of 6000 was considered reasonable. Data obtained at baseline (patient characteristics, hospitalization details, and anticoagulant therapy) were tabulated according to age (< 75 and ≥ 75 years) and renal function (creatinine clearance [CrCl] estimated using the Cockcroft–Gault formula: < 30 mL/min [severe impairment], 30 to < 50 mL/min [moderate impairment], 50 to < 80 mL/min [mild impairment], and ≥ 80 mL/min [normal]). The assignment of treatment choice was based on data from hospital discharge or 14 days after diagnosis (whichever was later). As such, patients who received parenteral anticoagulation prior to, or overlapping with, oral anticoagulation were considered to have been treated with the relevant oral anticoagulant.
Results
From January 13, 2016 to May 4, 2017, 6194 patients were consecutively enrolled from 34 countries, of whom 6095 patients were eligible. Ninety-nine patients were excluded: 46 patients had no documented VTE treatment; 29 had issues with the informed consent form; and 24 did not satisfy inclusion/exclusion criteria (lack of written informed consent, n = 3; lack of diagnosis of acute VTE/PE, n = 16; age < 18 years, n = 1; anticoagulation indicated for conditions other than VTE, n = 4).
Baseline characteristics and anticoagulation treatment of eligible patients according to age or renal function
Baseline characteristics are summarized in Table 1 for all 6095 eligible patients according to age, and for the 4203 patients who had CrCl data according to renal function. One-quarter of patients at baseline were aged ≥ 75 years, of whom nearly two-thirds were female. Of the 4507 patients diagnosed with DVT in the lower limb (with or without PE), 2819 (62.5%) had a proximal location (popliteal vein and above) and 2468 (54.8%) had a distal location (more than one location was possible). In patients aged < 75 years, 46.1% were female. A greater proportion of older patients (compared with those aged < 75 years) had renal impairment (mild 28.7% vs. 15.4%; moderate 21.4% vs. 2.9%; or severe 6.9% vs. 0.9%), PE as the index VTE event (32.4% vs. 23.9%), and comorbidities (73.9% vs. 58.1%), the most common being hypertension, diabetes mellitus, cancer, and a history of VTE.
Of the 4203 patients with known CrCl values, 38.2% had mild-to-moderate renal impairment (CrCl 30 to < 80 mL/min) and 3.5% had CrCl < 30 mL/min (Table 1). The percentage of male patients declined with decreasing renal function (55.8% for those with CrCl ≥ 80 mL/min to 30.4% for those with CrCl < 30 mL/min). With declining renal function from normal to moderate impairment, the proportion of patients with DVT alone appeared to decrease slightly (from 56.4% to 48.5%), and PE alone increased slightly (from 25.1% to 32.2%). The severe renal impairment group had fewer patients with both DVT and PE (10.1% compared with 17.8% to 19.3% in the other subgroups) and more with DVT alone (60.1% vs. 48.5% to 53.4%). More patients with mild, moderate, or severe renal impairment had comorbidities (75.9%, 80.9%, and 82.4%, respectively) compared with those with CrCl ≥ 80 mL/min (59.3%).
At the time of hospital discharge or 14 days after diagnosis (whichever was later), most patients were treated with NOACs (54.0%). VKAs were prescribed to about 1 in 5 patients, and a similar proportion received parenteral anticoagulation only. Age, above or below 75 years, had minimal impact on the anticoagulation prescription pattern (Fig. 1a). Use of NOACs decreased with worsening renal function, particularly in the subgroup with CrCl < 30 mL/min. The corollary was greater use of parenteral therapy alone (24.3% to 29.1% in the moderate and severe impairment groups vs. 15.6% to 16.6% in the normal and mild impairment groups) and greater use of VKAs (40.5% in the severe impairment group vs. 21.7% to 23.9% in the normal, moderate, and mild impairment groups) (Fig. 1b).
Pattern of anticoagulant use at hospital discharge or 14 days after diagnosis (whichever was later) according to a age or b renal function. aCrCl creatinine clearance, NOAC non-VKA oral anticoagulant, VKA vitamin K antagonist. aCrCl data missing for 1892 patients. CrCl estimated using the Cockcroft–Gault formula: < 30 mL/min represents severe impairment, 30 to < 50 mL/min moderate impairment, 50 to < 80 mL/min mild impairment, and ≥ 80 mL/min normal. b“Other” includes catheter-directed or systemic thrombolytic therapy
Baseline characteristics and anticoagulation treatment of patients with both age and renal function data available
Among the 4203 patients who had age and renal function data available, characteristics of the combined age/renal function subgroups (Table 2) generally reflected the patterns reported above for the separate age and renal function comparisons.
The prescription patterns of anticoagulants were generally similar between patients aged < 75 and ≥ 75 years who had normal or mild impairment of renal function. Notable differences were apparent between patients aged < 75 and ≥ 75 years who had moderately or severely impaired renal function (Fig. 2). Of those patients with moderate renal impairment aged ≥ 75 years, 56.1% received a NOAC, 19.4% a VKA, and 22.4% parenteral therapy only.
Pattern of anticoagulant use in 4203 patients with age and renal function data available (a). CrCl creatinine clearance, NOAC non-VKA oral anticoagulant, VKA vitamin K antagonist. aCrCl data missing for 1892 patients: 1389 patients aged < 75 years and 503 patients aged ≥ 75 years. CrCl estimated using the Cockcroft–Gault formula: < 30 mL/min represents severe impairment, 30 to < 50 mL/min moderate impairment, 50 to < 80 mL/min mild impairment, and ≥ 80 mL/min normal. b“Other” includes catheter-directed or systemic thrombolytic therapy
Discussion
We observed that older patients and those with renal impairment were more often female and more likely to have comorbidities than younger patients or those with normal renal function. Up to 14 days after diagnosis, or by the time of hospital discharge, more than half the patients had been treated with NOACs and over one-fifth were prescribed VKAs. Surprisingly, the proportions of patients receiving NOACs and VKAs were almost the same for the elderly and non-elderly. As expected, NOAC use decreased with worsening renal function, whereas the proportion treated with VKAs tended to increase as renal function declined. It was unexpected, however, that the decrease in the use of NOACs occurred more markedly in younger patients with renal impairment than in elderly patients with renal impairment. Also, the use of parenteral therapy alone increased in patients with moderate and severe renal impairment as compared to patients with normal renal function or mild impairment. This finding is partially unexpected since caution is also recommended with the use of low-molecular-weight heparin in patients with severe renal insufficiency and with the use of fondaparinux in patients with moderate and severe renal insufficiency, due to the renal excretion of these drugs [13]. We note a limitation of the study, that CrCl values were missing for approximately one-third of patients. Data from this and other observational studies show that creatinine levels or estimation of creatinine clearance are not routinely available in patients with VTE. For example, CrCl values were missing for approximately one-fifth of patients in the observational GARFIELD-VTE study cohort [14].
In the real-world setting, patient characteristics and disease management may differ from those in RCTs. The proportions of patients at baseline in RE-COVERY DVT/PE who were aged ≥ 75 years (25.3%) or who had mild-to-moderate renal impairment (38.2%) were greater than those recruited in RCTs of NOACs for acute VTE treatment (~ 12–13% and ~ 26%, respectively) (Table 3) [4, 15, 16]. These RCT data included a pooled analysis of the phase III RE-COVER and RE-COVER II trials of dabigatran versus warfarin analyzed by age and renal function subgroups. In another observational study, GARFIELD-VTE [14], the proportion of patients with CrCl 30–89 mL/min was 41.5% of those with available CrCl estimates. However, fewer patients (18.1%) in the GARFIELD-VTE registry than in RE-COVERY DVT/PE were aged ≥ 75 years (Table 3).
A key consideration when prescribing any drug in the elderly and in those with impaired renal function, particularly anticoagulants, is reduced drug clearance, as excessive anticoagulation can increase the risk of major bleeding [13, 17]. However, older age [1] and decreased CrCl [18] are also associated with an increased risk of recurrent VTE. Therefore, provision of anticoagulation to these patient groups is challenging. The age and renal function subgroup data from RCTs in VTE show that the effects of NOACs relative to warfarin on safety and efficacy outcomes are consistent with the effects in the entire study population [4,5,6,7, 15, 16, 19,20,21].
Overall, 54.0% of patients in our study were prescribed a NOAC as their oral anticoagulant therapy assessed at 14 days after diagnosis or hospital discharge (whichever was sooner)—greater than the proportion in GARFIELD-VTE (48.7%). Among RE-COVERY DVT/PE patients with moderate renal impairment, about one-third of those aged < 75 years received NOACs. In contrast, more than half of those aged ≥ 75 years were treated with NOACs. The reasons for the different patterns of prescribing among elderly and young patients with renal impairment remain uncertain. However, we speculate that physicians consider that renal impairment is an expected component of frailty among elderly patients, and that the safer profile of NOACs justifies their use despite renal disease. The available subgroup data from RCTs in VTE appear to support this approach [4,5,6,7, 15, 16, 19,20,21], with meta-analyses showing consistent safety and efficacy of NOACs versus VKAs in subgroups including moderate renal impairment and age ≥ 75 years [22, 23].
In summary, the population treated for acute VTE in routine clinical practice includes more elderly and renally impaired patients than those represented in RCTs. Decreasing renal function, particularly in the subgroup with CrCl < 30 mL/min, but not older age, was associated with less use of NOACs. Nevertheless, more than half of the older patients with moderate renal impairment received a NOAC. These baseline data from RE-COVERY DVT/PE provide insight into patient characteristics and how age and renal function are related to patterns of anticoagulant therapy.
Availability of data and material
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfill their role and obligations as authors under the International Committee of Medical Journal Editors criteria.
Furthermore, clinical study documents (e.g., study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/transparency_policy.html.
Prior to providing access, documents will be examined and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study participants.
Clinical study reports and related clinical documents can be requested via this link: https://trials.boehringer-ingelheim.com/trial_results/clinical_submission_documents.html. All such requests will be governed by a Document Sharing Agreement.
Bona fide, qualified scientific and medical researchers may request access to de-identified, analyzable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a Data Sharing Agreement, data are shared in a secured data-access system for a limited period of 1 year, which may be extended upon request.
Researchers should use https://clinicalstudydatarequest.com to request access to study data.
References
Spencer FA, Gore JM, Lessard D, Emery C, Pacifico L, Reed G, Gurwitz JH, Goldberg RJ (2008) Venous thromboembolism in the elderly. A community-based perspective. Thromb Haemost 100(5):780–788. https://doi.org/10.1160/TH08-04-0255
Hughes S, Szeki I, Nash MJ, Thachil J (2014) Anticoagulation in chronic kidney disease patients-the practical aspects. Clin Kidney J 7(5):442–449. https://doi.org/10.1093/ckj/sfu080
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149(2):315–352. https://doi.org/10.1016/j.chest.2015.11.026
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, Weitz JI, AMPLIFY Investigators (2013) Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 369(9):799–808. https://doi.org/10.1056/NEJMoa1302507
Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S, for the EINSTEIN Investigators (2010) Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 363(26):2499–2510. https://doi.org/10.1056/NEJMoa1007903
Einstein-PE Investigators, Buller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A, Berkowitz SD, Bounameaux H, Davidson BL, Misselwitz F, Gallus AS, Raskob GE, Schellong S, Segers A (2012) Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 366(14):1287–1297. https://doi.org/10.1056/NEJMoa1113572
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ (2009) Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 361(24):2342–2352. https://doi.org/10.1056/NEJMoa0906598
European Medicines Agency. Dabigatran. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf. Accessed 6 Apr 2020
European Medicines Agency. Edoxaban. Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002629/WC500189045.pdf. Accessed 6 Apr 2020
Electronic Medicines Compendium. Rivaroxaban. Summary of product characteristics. https://www.medicines.org.uk/emc/medicine/25586. Accessed 6 Apr 2020
Goldhaber SZ, Ageno W, Casella IB, Chee KH, Schellong S, Singer DE, Desch M, Reilly PA, Donado E, Tang W, Voccia I, Schulman S (2020) Profile of patients diagnosed with acute venous thromboembolism in routine clinical practice: the RE-COVERY DVT/PE™ study. Am J Med 133(8):936–945 https://doi.org/10.1016/j.amjmed.2020.03.036
Ageno W, Casella IB, Han CK, Raskob GE, Schellong S, Schulman S, Singer DE, Kimura K, Tang W, Desch M, Goldhaber SZ (2017) RE-COVERY DVT/PE: rationale and design of a prospective observational study of acute venous thromboembolism with a focus on dabigatran etexilate. Thromb Haemost 117(2):415–421. https://doi.org/10.1160/TH16-07-0566
Cook LM, Kahn SR, Goodwin J, Kovacs MJ (2007) Frequency of renal impairment, advanced age, obesity and cancer in venous thromboembolism patients in clinical practice. J Thromb Haemost 5(5):937–941. https://doi.org/10.1111/j.1538-7836.2007.02507.x
Goldhaber SZ, Schulman S, Eriksson H, Feuring M, Fraessdorf M, Kreuzer J, Schuler E, Schellong S, Kakkar A (2017) Dabigatran versus warfarin for acute venous thromboembolism in elderly or impaired renal function patients: pooled analysis of RE-COVER and RE-COVER II. Thromb Haemost 117(11):2045–2052. https://doi.org/10.1160/TH17-03-0176
Hokusai VTE Investigators, Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, Raskob GE, Schellong SM, Schwocho L, Segers A, Shi M, Verhamme P, Wells P (2013) Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 369(15):1406–1415. https://doi.org/10.1056/NEJMoa1306638
Ageno W, Haas S, Weitz JI, Goldhaber SZ, Turpie AGG, Goto S, Angchaisuksiri P, Nielsen JD, Kayani G, Pieper KS, Schellong S, Bounameaux H, Mantovani LG, Prandoni P, Kakkar AK (2019) Characteristics and management of patients with venous thromboembolism: the GARFIELD-VTE Registry. Thromb Haemost 119(2):319–327. https://doi.org/10.1055/s-0038-1676611
Lopez-Jimenez L, Montero M, Gonzalez-Fajardo JA, Arcelus JI, Suarez C, Lobo JL, Monreal M, Investigators RIETE (2006) Venous thromboembolism in very elderly patients: findings from a prospective registry (RIETE). Haematologica 91(8):1046–1051
Rattazzi M, Villalta S, De Lucchi L, Sponchiado A, Galliazzo S, Faggin E, Pagliara V, Zilli C, Callegari E, Caberlotto L, Puato M, Pauletto P (2017) Chronic kidney disease is associated with increased risk of venous thromboembolism recurrence. Thromb Res 160:32–37. https://doi.org/10.1016/j.thromres.2017.10.011
Bauersachs RM, Lensing AW, Prins MH, Kubitza D, Pap AF, Decousus H, Beyer-Westendorf J, Prandoni P (2014) Rivaroxaban versus enoxaparin/vitamin K antagonist therapy in patients with venous thromboembolism and renal impairment. Thromb J 12:25. https://doi.org/10.1186/1477-9560-12-25
Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, Christiansen AV, Friedman J, Le Maulf F, Peter N, Kearon C, Re-Cover II Trial Investigators (2014) Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 129(7):764–772. https://doi.org/10.1161/CIRCULATIONAHA.113.004450
Verhamme P, Wells PS, Segers A, Ageno W, Brekelmans MP, Cohen AT, Meyer G, Grosso MA, Raskob G, Weitz JI, Zhang G, Buller H (2016) Dose reduction of edoxaban preserves efficacy and safety for the treatment of venous thromboembolism. An analysis of the randomised, double-blind HOKUSAI VTE trial. Thromb Haemost 116(4):747–753. https://doi.org/10.1160/th16-03-0244
Gomez-Outes A, Terleira-Fernandez AI, Lecumberri R, Suarez-Gea ML, Vargas-Castrillon E (2014) Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res 134(4):774–782. https://doi.org/10.1016/j.thromres.2014.06.020
van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR (2014) Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 124(12):1968–1975. https://doi.org/10.1182/blood-2014-04-571232
Acknowledgements
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Keith Day, PhD, of Parexel, during the preparation of this article. The authors thank the patients who participated in this trial, and their families, as well as the investigators, study coordinators, study teams, and nurses.
Funding
Open access funding provided by Università degli Studi dell'Insubria within the CRUI-CARE Agreement. The RE-COVERY DVT/PE study was supported by Boehringer Ingelheim.
Author information
Authors and Affiliations
Contributions
Dr W Ageno was responsible for the concept of the secondary analyses presented in this manuscript. Dr W Tang was responsible for the data analysis. All authors were responsible for the study concept and design, interpretation of the data, preparation, review or approval of the manuscript, revision of intellectual content, and the decision to submit the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ageno has participated in advisory boards for Bayer, Portola, Aspen, Sanofi, Daiichi Sankyo, Boehringer Ingelheim, and has received travel or research support from Bayer, Portola, Aspen, Janssen, Sanofi, Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, and Boehringer Ingelheim. Dr. Casella has received speaker and/or consultancy fees from Boehringer Ingelheim, Bayer, Daiichi Sankyo, Pfizer, and Amgen. Dr. Chee has received speaker fees from Boehringer Ingelheim, Bristol-Myers Squibb, and Pfizer. Dr. Schellong has received speaker fees from Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Sanofi, and LEO Pharma. He has received consultancy fees from Bayer HealthCare, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, and Sanofi. Dr. Schulman has received honoraria from Alnylam, Boehringer Ingelheim, Bayer HealthCare, Daiichi Sankyo, Pfizer, and Sanofi, and research support from Boehringer Ingelheim and Octapharma. Dr. Singer has received honoraria from Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Johnson & Johnson, and Pfizer, and research support from Boehringer Ingelheim and Bristol-Myers Squibb. Dr. Desch, Dr. Tang, Dr. Voccia, and Dr. Zint are employees of Boehringer Ingelheim. Dr. Goldhaber has received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Boston Scientific BTG, Daiichi Sankyo, Janssen, and the US National Heart Lung and Blood Institute. He is a consultant for Bayer and Boehringer Ingelheim.
Ethical approval
The study was carried out in compliance with the protocol and the principles laid down in the Declaration of Helsinki. In addition, the applicable sections of the guidelines for Good Clinical Practice, Good Epidemiological Practice, and Good Pharmacoepidemiology Practices, and local regulations were followed.
Informed consent
Patients (or their legal representative) provided written informed consent before study entrance, in accordance with local regulations. No study procedures or data recording were performed unless a patient had consented to participate in the study or a waiver had been obtained in accordance with local regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of the article was revised to add the funding note: Open access funding provided by Università degli Studi dell'Insubria within the CRUI-CARE Agreement.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ageno, W., Casella, I.B., Chee, K.H. et al. Profile of patients diagnosed with acute venous thromboembolism in routine practice according to age and renal function: RE-COVERY DVT/PE study. J Thromb Thrombolysis 51, 561–570 (2021). https://doi.org/10.1007/s11239-020-02239-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02239-9