Abstract
In patients with active cancer and acute venous thromboembolism (VTE), the low-molecular-weight-heparin (LMWH) dalteparin is more effective than vitamin K antagonist (VKA) in reducing the risk of recurrent venous thromboembolism (rVTE) without increasing the risk of bleeding. However, the relative benefit of LMWH versus VKA in patients with active cancer at high or low risk of rVTE and bleeding is unclear. This post hoc analysis used data from the CLOT study to explore the efficacy and safety of LMWH versus VKA in preventing recurrent thrombosis in high- and low-risk patients with active cancer. High-risk patients were defined by metastatic disease and/or antineoplastic treatment at baseline; low-risk patients presented with neither. Among high-risk patients, rVTE occurred in 25/318 (8%) (LMWH) versus 53/314 (17%) (VKA) (hazard ratio, 0.44; p = 0.001). No significant difference was detected in the rate of major or any bleeding. The 6-month mortality rate was 40% (LMWH) versus 41% (VKA). In low-risk patients, 2/20 (10%) (LMWH) had rVTE versus 0/24 (0%) (VKA) (hazard ratio, not estimable; p = 0.998). No significant difference was detected in the rate of major or any bleeding. The 6-month mortality rate was 20% (LMWH) versus 29% (VKA). In patients with cancer-associated thrombosis at high risk of rVTE and bleeding, the LMWH dalteparin was more effective than VKA in reducing the risk of rVTE without increasing the risk of bleeding. No difference in rate of rVTE or bleeding was observed between LMWH and VKA among low-risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Patients with cancer-associated thrombosis are heterogeneous. Substantial differences in clinical risk factors between these patients may lead to different clinical outcomes despite therapeutic anticoagulation with the same agent.
-
The relative benefit of low-molecular-weight-heparin (LMWH) in patients with cancer-associated thrombosis at different levels of risk for recurrent VTE (rVTE) and bleeding is unclear.
-
This exploratory post hoc analysis of the pivotal CLOT trial compared the efficacy and safety of LMWH with that of vitamin K antagonist (VKA) in subgroups of patients with active cancer at high risk or low risk of recurrent thrombosis and bleeding.
-
In patients with cancer-associated thrombosis at high risk of rVTE and bleeding, LMWH was more effective than VKA in reducing the risk of rVTE without increasing the risk of bleeding. However, no difference in efficacy or safety was observed between LMWH and VKA among low-risk patients.
-
Future prospective studies are needed to further define the relative efficacy and safety of different anticoagulation treatments in patients with cancer-associated thrombosis and different levels of risk for rVTE and bleeding.
Background
Current standard treatment for acute venous thromboembolism (VTE) in patients with active cancer is long-term low-molecular-weight-heparin (LMWH) [1, 2]. This recommendation is based primarily on the ‘Comparison of Low-Molecular-Weight-Heparin vs Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT)’ study which showed an overall 52% relative risk reduction in the rate of symptomatic, recurrent venous thromboembolism (rVTE) over 6 months with the LMWH dalteparin versus oral vitamin K antagonist (VKA) therapy without an increased risk of bleeding [3]. This approach is effective in most patients, but patients with active cancer are heterogeneous. There are substantial differences in risk for rVTE and hemorrhagic complications because of differences in clinical risk factors [4]. Individual risk factors for rVTE in cancer cohorts include locally advanced or metastatic cancer, antineoplastic treatment, primary tumor site, and recent diagnosis of malignancy [2,3,4,5,6]. Key risk factors associated with an increased risk of major bleeding include metastatic disease, a recent bleeding event, and renal insufficiency [4]. Importantly, substantial differences in clinical risk factors between patients with cancer-associated thrombosis may lead to different clinical outcomes despite therapeutic anticoagulation with the same agent. As a result, the relative benefit of LMWH versus VKA in patients with cancer-associated thrombosis at high or low risk of rVTE and bleeding is unclear.
We performed an exploratory, post hoc analysis of the CLOT study to compare the efficacy and safety of the LMWH dalteparin versus VKA in subgroups of patients with active cancer at high risk or low risk of rVTE and bleeding.
Methods
Study design and population
A detailed description of the CLOT study design, population, treatment regimens, and outcome measures has been published previously [3]. The CLOT study was conducted in compliance with the ethical principles originating in, or derived from, the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice Guidelines. All patients provided written, informed consent.
This exploratory post hoc analysis of data from the CLOT study compared the efficacy and safety of subcutaneous LMWH with that of oral VKA therapy in preventing rVTE in patients with active cancer at high or low risk of recurrent thrombosis and bleeding. High-risk patients were empirically defined as those with known metastatic disease and/or were receiving recent antineoplastic treatment (chemotherapy, radiation, and/or surgery 6 months prior to or at randomization) at baseline. These parameters, readily determined in clinical practice, are established independent risk factors for rVTE and bleeding during therapeutic anticoagulation [4]. Low-risk patients excluded high-risk patients as defined above, and therefore, included patients with neither metastatic disease nor antineoplastic treatment at baseline.
LMWH patients were administered 200 IU of dalteparin per kilogram once daily for 30 days, with a maximum daily dose of 18,000 IU. For the remaining 5 months, dalteparin was administered at a dose of approximately 150 IU per kilogram once daily. If the platelet count fell to 50,000–100,000 per microliter during treatment, the dalteparin dose was temporarily reduced.
Oral VKA patients initially received 200 IU of dalteparin per kilogram (maximal daily dose, 18,000 IU) once daily and, within 24 h, a vitamin K antagonist for 6 months. Dalteparin was discontinued after a minimum of 5 days and once the international normalized ratio (INR) remained between 2.0 and 3.0 for 2 consecutive days. All VKA doses were adjusted to reach a target INR of 2.5 (therapeutic range 2.0–3.0). Target INR was reduced to 2.0 (range 1.5–2.5) if the platelet count fell to 50,000–100,000 per microliter during treatment. In all patients, treatment was to continue for 6 months.
Outcome measures
The primary efficacy outcome was the first episode of objectively diagnosed, symptomatic rVTE, including deep-vein thrombosis, pulmonary embolism, or both, during the 6-month study period in the intention-to-treat population (ITT; all randomized patients in the high- and low-risk patient subgroups).
Secondary outcomes included clinically overt bleeding during the 6-month study period (both any and major) and death. Major bleeding events were defined by an overt bleeding event associated with a fall in hemoglobin ≥ 2.0 g/dL, the need for a transfusion of ≥ 2 units of blood, involvement of a critical site (retroperitoneal, intracranial, intraspinal, intraocular, or pericardial areas), or an event leading to death. Any bleeding event also included all other overt hemorrhagic events that did not meet the criteria for classification as major bleeding. The safety analyses included patients who received at least one treatment dose.
All suspected rVTE and bleeding events were objectively reviewed and verified by an independent Central Adjudication Committee blinded to treatment allocation.
Statistical analysis
Descriptive statistics were provided for baseline characteristics, tumor and VTE history, and risk factors. Time to first event analyses were conducted for the high-risk group using the Kaplan–Meier method and data were compared using the log-rank test with a 5% significance level. Cox proportional hazard regression models were employed to assess the effect of treatment (LMWH versus VKA) and other potential prognostic factors on VTE recurrence, bleeding, and death events in the high-risk group. Time to first event analyses and multivariate analyses were not conducted for the low-risk group due to the small sample size (n = 44). Competitive risk analysis for death was not performed in either group due to similar mortality rates between treatment arms [7]. Significance was set at the 5% level. No statistical adjustments for multiple testing were made.
Results
Study population
Baseline patient demographic and clinical characteristics stratified by risk (high/low) and treatment are shown in Table 1. Overall, 632/676 (93%) of patients in CLOT had metastatic disease and/or recent antineoplastic treatment at baseline (LMWH arm, 318; VKA arm, 314). Only 44/676 (7%) had neither metastatic disease nor recent antineoplastic treatment at baseline and were classified in the low-risk group (LMWH arm, 20; VKA arm, 24). In the high-risk subgroup, baseline demographics and clinical characteristics were similar between treatment arms. In the low-risk subgroup there was an imbalance in select baseline factors between treatment arms; however, no statistical comparisons were made because of the small number of patients.
VTE recurrence
First episodes of rVTE were determined in the ITT population according to treatment and risk subgroups (Fig. 1). Overall, 25/318 (8%) LMWH-treated patients and 53/314 (17%) VKA-treated patients in the high-risk group experienced ≥ 1 rVTE during the 6-month study period (hazard ratio [HR] 0.44; 95% confidence interval [CI] 0.273–0.708; p = 0.001). In contrast, 2/20 (10%) LMWH-treated patients and 0/24 (0%) VKA-treated patients in the low-risk group experienced ≥ 1 rVTE during the 6-month study period (HR; 95% CI, not estimable; p = 0.998).
Comparison of treatment effects on the first VTE recurrence, first any bleeding, first major bleed, and death events in high- and low-risk patient subgroups. *Not estimable. aCox proportional model with treatment as covariate. bIntention-to-treat patients. cAs-treated patients. Statistically significant p values (p < 0.05) are shown in bold text. CI confidence interval, VKA vitamin K antagonist, VTE venous thromboembolism, High-risk metastatic disease and/or antineoplastic treatment, low-risk no metastatic disease and no neoplastic treatment
Kaplan–Meier curves showing time to first rVTE at 6 months for high-risk patients in the LMWH and VKA treatment groups are presented in Fig. 2.
Kaplan–Meier estimates of the time to first VTE and bleeding events in high-risk patients. a Time to first VTE reccurence at 6 monthsab Time to first any bleeding event at 6 monthsbc Time to first major bleeding event at 6 monthsb. Significance set at 5% and p-value calculated using log-rank test. aITT population. bAST population. AST as-treated, ITT intention-to treat, VKA vitamin K antagonist, VTE venous thromboembolism
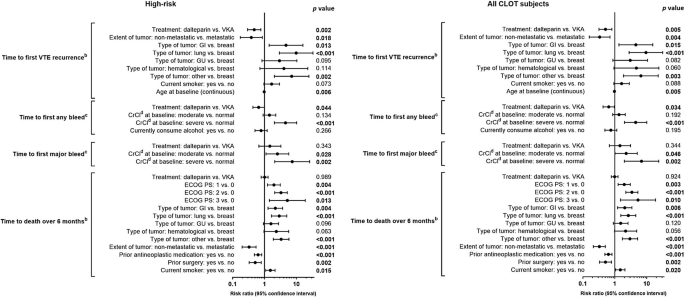
In the high-risk group, Cox proportional hazards regression analyses demonstrated that treatment with LMWH lowered the risk of rVTE versus VKA. This reduction remained statistically significant after adjustment for other prognostic factors (risk ratio [RR] 0.47; 95% CI 0.293–0.764; p = 0.002; Fig. 3). Additionally, significant interactions between treatment group and risk factors were detected. The risk of rVTE was higher in patients with metastatic versus non-metastatic cancer (p = 0.018) and in younger patients versus older patients (p = 0.006). Notably, the risk of rVTE was not higher in patients with versus without recent antineoplastic treatment in the high-risk patient group.
Time to first event data following adjustment for potential prognostic factorsa assessed at study entry. aPrognostic variables applied to CLOT study. With the exception of treatment effect, only factors significant in a univariate analysis at the 0.10 significance level were considered in the process of building the final multivariate model. bIntention-to-treat population. cAs-treated population. dCrCl values were calculated using the Cockcroft-Gault formula. Normal: CrCl ≥ 60 mL/min; moderate impairment: 30 ≤ CrCl < 60 mL/min; severe impairment: CrCl < 30 mL/min. High-risk metastatic disease and/or antineoplastic treatment. Statistically significant p values (p < 0.05) are shown in bold text. CI confidence interval, CrCl creatinine clearance, ECOG PS Eastern Cooperative Oncology Group performance status, GI gastrointestinal, GU genitourinary, VKA vitamin K antagonist, VTE venous thromboembolism
Bleeding events
First instances of any or major bleeding were determined in the as-treated population according to treatment and subpopulation (Fig. 1). The proportion of patients in the high-risk group experiencing ≥ 1 any bleeding episode was lower in the LMWH arm than in the VKA arm: 44/318 (14%) versus 57/311 (18%), respectively, but this difference was not statistically significant (HR 0.71; 95% CI 0.476–1.047; p = 0.083). The proportion of patients in the low-risk group experiencing ≥ 1 any bleeding event was also lower in LMWH-treated patients (2/20 [10%]) versus VKA-treated patients (5/24 [21%]). This difference was not statistically significant (HR 0.42; 95% CI 0.080–2.160; p = 0.296).
A greater proportion of high-risk patients experienced ≥ 1 major bleeding episode while receiving LMWH (18/318 [6%]) than VKA (11/311 [4%]). However, between-treatment differences were not statistically significant (HR 1.51; 95% CI 0.711–3.188; p = 0.285). The rate of major bleeding episodes in the low-risk group was similar between LMWH- and VKA-treated patients (1/20 [5%] vs. 1/24 [4%], respectively).
Kaplan–Meier curves estimating time to first any or major bleeding event over 6 months for high-risk patients are presented in Fig. 1. The cumulative probability of any or major bleeding event over 6 months for LMWH versus VKA was not statistically significant (2-sided log-rank test: any bleeding event, p = 0.082; major bleeding event, p = 0.282).
Cox proportional hazards models for the high-risk group demonstrated a statistically significant lowered risk of any bleeding event for LMWH versus VKA after adjustment for other prognostic factors for bleeding (RR 0.65; 95% CI 0.431–0.989; p = 0.044; Fig. 3). Additionally, the risk of any bleeding was significantly lower in patients with normal renal function versus severe renal impairment (creatinine clearance [CrCl] < 30 mL/min) (p < 0.001), a finding comparable with the full CLOT study [8]. The larger proportion of high-risk patients experiencing ≥ 1 major bleeding event with LMWH versus VKA remained non-significant when the model was adjusted for other prognostic factors for bleeding (RR 1.47; 95% CI 0.664–3.243; p = 0.343). The risk of major bleeding events was higher in patients with moderate (30 < CrCl < 60 mL/min) (p = 0.028) or severe renal impairment (p = 0.002) versus normal renal function. Neither metastatic disease alone nor recent antineoplastic treatment alone increased the rate of any or major bleeding events in this high-risk patient group.
Death events
The death rate among high-risk patients during the 6-month study period was 127/318 (40%) in LMWH-treated patients and 130/314 (41%) in VKA-treated patients. Among low-risk patients, 6-month mortality was lower: 4/20 (20%) in the LMWH group and 7/24 (29%) in the VKA group.
In the high-risk group, Cox proportional hazards models, adjusted for other factors prognostic for death, demonstrated similar 6-month mortality rates between LMWH and VKA groups (RR 1.00; 95% CI 0.781–1.285; p = 0.989; Fig. 3). Metastatic disease was found to increase 6-month mortality rates in this high-risk patient group.
Discussion
To our knowledge, this exploratory, post hoc analysis is the first to study the efficacy and safety of LMWH versus VKA for the treatment of acute VTE in patients with active cancer at high or low risk of rVTE and bleeding complications. High-risk patients, 93% of the total CLOT population, were defined as having metastatic disease and/or receiving recent antineoplastic treatment at baseline, while low-risk patients had neither. These definitions were empirically derived, based on prior reports and clinical experience that metastatic disease and active cancer therapy are risk factors for recurrent thrombosis and bleeding. Our analysis showed that LMWH treatment for up to 6 months in high risk patients was more effective than VKA in reducing the risk of recurrent thromboembolism without increasing the risk of bleeding. At 6 months, the overall risk of recurrent thrombosis and major bleeding was 12% and 5%, respectively. Importantly, the 8% rate of rVTE at 6 months in the high-risk LMWH group of the CLOT study is consistent with the rate reported recently for LMWH in the DALTECAN Study [9].
Among high-risk patients, there was a higher risk of rVTE in patients with metastatic versus non-metastatic cancer, which supports the empirical use of baseline metastatic cancer to define high-risk patients. Interestingly, the risk of rVTE was not higher in patients who received recent antineoplastic treatment versus those without such treatment. Together, these findings suggest that baseline metastatic disease may contribute more to increased risk of recurrent thrombosis than recent antineoplastic treatment in high-risk patients. Neither metastatic disease nor recent antineoplastic treatment was found to increase the rate of any or major bleeding events in the high-risk patient group. As a result, the risk of hemorrhagic complications under therapeutic anticoagulation may be more dependent on other risk factors than baseline metastatic disease or antineoplastic treatment. Recent findings from randomized trials comparing dalteparin with a direct oral anticoagulant (DOAC) [10, 11], indicate that choice of anticoagulant is an important predictor of bleeding, and that DOAC therapy may not be the optimal treatment for cancer-associated thrombosis among those patients with risk factors for bleeding.
Treatment with LMWH in patients with active cancer at low risk of rVTE and bleeding complications showed similar rates of rVTE and bleeding with VKA. These results should be interpreted with caution due to the small number of subjects and imbalance of prognostic factors (eg, age, tumor type and site, hospitalization) in the low-risk treatment group. However, our results are consistent with the CATCH trial, which showed that the LMWH tinzaparin was not more effective than VKA in preventing rVTE [12]. Although the CATCH trial used eligibility criteria similar to CLOT, it had fewer patients with metastatic disease and/or receiving anticancer therapy and better overall survival. Similar findings have also been reported in previous trials comparing LMWH and VKA for secondary prophylaxis of VTE in patients without cancer who are at low risk of recurrent events and hemorrhagic complications [13, 14]. Consequently, patients with less advanced cancer or better prognosis who are at lower risk of rVTE may not experience the same benefit with LMWH as those with metastatic malignancies.
Data to date show that there are substantial and clinically relevant differences in baseline patient characteristics and prognosis among various clinical trials (Table 2). This heterogeneity is an important consideration for cross-study comparisons and network meta-analyses in determining optimal anticoagulant therapy. For example, compared with high-risk CLOT patients, fewer patients with cancer in the DOAC-VKA [15,16,17], CATCH [12], and Hokusai VTE Cancer (edoxaban versus dalteparin) [10] trials had metastatic disease at baseline. Fewer patients in both the CATCH and Hokusai VTE Cancer trials had recent antineoplastic therapy at baseline or an Eastern Cooperative Oncology Group performance status of 2. Most importantly, all-cause mortality was lower in patients with cancer in DOAC-VKA trials, CATCH, and Hokusai VTE Cancer trials than in high-risk patients enrolled in CLOT. Notably, 6-month overall mortality was 26% in Hokusai VTE Cancer but 41% in high-risk CLOT. Despite a decade gap between these two trials, it is unlikely that differences in cancer therapeutics alone are responsible for the observed difference in mortality. As a result, patients with cancer in the DOAC-VKA, CATCH, and Hokusai VTE Cancer trials had better prognosis, with lower thrombotic and hemorrhagic risks, than overall and high-risk patients in CLOT. These findings are a strong reminder that extrapolation of clinical outcomes from one patient cohort to another may not be valid and should be avoided.
Overall, our results suggest that patients with cancer-associated thrombosis have different levels of risk for rVTE and bleeding which can lead to different clinical outcomes despite therapeutic anticoagulation with the same agent. As a result, clinicians must be mindful of patient and cancer characteristics when considering the choice of anticoagulant therapy. Our results, combined with recent clinical trials, suggest that patients with active cancer at high risk of rVTE and, particularly, bleeding are likely to benefit more from therapeutic anticoagulation with LMWH, whereas lower-risk patients can choose among LMWH, DOAC, and warfarin. Individual patient benefit-risk assessment and preference should be essential components of the therapeutic decision.
Interpretation of this post hoc analysis has limitations. First, CLOT did not stratify patients by specific risk factors, and was not powered to detect between-treatment differences in subgroup analyses [3]. However, we used a priori documented baseline characteristics that have been established as important risk factors for thrombosis and bleeding. Second, we did not examine other risk factors such as biomarkers. Our approach was to use easily identifiable features for simple risk stratification. Finally, patient numbers in the low-risk non-metastatic disease/non-antineoplastic treatment subgroup were small, thus limiting the validity and value of our findings for this subgroup.
Conclusions
Among patients with active cancer and acute VTE at high risk of recurrent thrombosis and bleeding, long-term self-injection of LMWH was more effective than oral VKA in reducing the risk of rVTE without increasing the risk of bleeding. In contrast, no difference in rate of rVTE or bleeding was observed between LMWH and VKA in low-risk cancer patients.
References
Kearon C, Akl EA, Ornelas J et al (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149:315–352. https://doi.org/10.1016/j.chest.2015.11.026
Lyman GH, Bohlke K, Khorana AA et al (2015) Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol 33:654–656. https://doi.org/10.1200/JCO.2014.59.7351
Lee AY, Levine MN, Baker RI et al (2003) Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 349:146–153. https://doi.org/10.1056/NEJMoa025313
Menapace LA, McCrae KR, Khorana AA (2016) Predictors of recurrent venous thromboembolism and bleeding on anticoagulation. Thromb Res 140 (Suppl 1):S93–S98. https://doi.org/10.1016/S0049-3848(16)30106-2
Chee CE, Ashrani AA, Marks RS, Petterson TM, Bailey KR, Melton LJ III, Heit JA (2014) Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood 123:3972–3978. https://doi.org/10.1182/blood-2014-01-549733
Lee AYY (2017) When can we stop anticoagulation in patients with cancer-associated thrombosis? Blood 130:2484–2490. https://doi.org/10.1182/blood-2017-05-787929
Parpia S, Julian JA, Thabane L, Lee AY, Rickles FR, Levine MN (2011) Competing events in patients with malignant disease who are at risk for recurrent venous thromboembolism. Contemp Clin Trials 32:829–833. https://doi.org/10.1016/j.cct.2011.07.005
Woodruff S, Feugère G, Abreu P, Heissler J, Ruiz MT, Jen F (2016) A post hoc analysis of dalteparin versus oral anticoagulant (VKA) therapy for the prevention of recurrent venous thromboembolism (rVTE) in patients with cancer and renal impairment. J Thromb Thrombolysis 42:494–504. https://doi.org/10.1007/s11239-016-1386-8
Francis CW, Kessler CM, Goldhaber SZ et al (2015) Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost 13:1028–1035. https://doi.org/10.1111/jth.12923
Raskob GE, van Es N, Verhamme P et al (2018) Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 378:615–624. https://doi.org/10.1056/NEJMoa1711948
Young AM, Marshall A, Thirlwall J et al (2018) Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 36:2017–2023. https://doi.org/10.1200/JCO.2018.78.8034
Lee AYY, Kamphuisen PW, Meyer G, Bauersachs R, Janas MS, Jarner MF, Khorana AA (2015) Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA 314:677–686. https://doi.org/10.1001/jama.2015.9243
Das SK, Cohen AT, Edmondson RA, Melissari E, Kakkar VV (1996) Low-molecular-weight heparin versus warfarin for prevention of recurrent venous thromboembolism: a randomized trial. World J Surg 20:521–526
Lopaciuk S, Bielska-Falda H, Noszczyk W et al (1999) Low molecular weight heparin versus acenocoumarol in the secondary prophylaxis of deep vein thrombosis. Thromb Haemost 81:26–31
Prins MH, Lensing AW, Brighton TA et al (2014) Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 1:e37–e46. https://doi.org/10.1016/S2352-3026(14)70018-3
Schulman S, Goldhaber SZ, Kearon C, Kakkar AK, Schellong S, Eriksson H, Hantel S, Feuring M, Kreuzer J (2015) Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost 114:150–157. https://doi.org/10.1160/TH14-11-0977
Raskob GE, van Es N, Segers A et al (2016) Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol 3:e379–e387. https://doi.org/10.1016/S2352-3026(16)30057-6
Acknowledgements
Medical writing support was provided by Katy Beck, PhD of Engage Scientific Solutions (Horsham, UK) and funded by Pfizer.
Funding
This study was sponsored by Pfizer. Medical writing support was provided by Katy Beck, PhD of Engage Scientific Solutions (Horsham, UK) and funded by Pfizer.
Author information
Authors and Affiliations
Contributions
All authors were involved in the design of the analyses, the analysis and interpretation of the data, drafted and critically revised the manuscript, read and approved the final draft, and agree to be accountable for all aspects of the data.
Corresponding author
Ethics declarations
Conflict of interest
AL has provided consultancy for Pfizer, LEO Pharma, and Bayer, has received honoraria from Pfizer, and research funding from BMS. MC has provided consultancy for Pfizer, Leo Pharma, Bayer, and BMS, and received research funding from BMS, Leo Pharma, and Pfizer. SW, GF, and PA are employees of Pfizer and hold stock and/or stock options in Pfizer. JH was an employee of Pfizer and held stock and/or stock options in Pfizer at the time of study conduct.
Ethical approval
The CLOT study was conducted in compliance with the ethical principles originating in, or derived from, the Declaration of Helsinki and in compliance with all International Conference on Harmonisation Good Clinical Practice Guidelines.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Woodruff, S., Lee, A.Y.Y., Carrier, M. et al. Low-molecular-weight-heparin versus a coumarin for the prevention of recurrent venous thromboembolism in high- and low-risk patients with active cancer: a post hoc analysis of the CLOT Study. J Thromb Thrombolysis 47, 495–504 (2019). https://doi.org/10.1007/s11239-019-01833-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01833-w