Abstract
Acute pulmonary embolism (PE) is a frequent cause of death and serious disability. The risk of PE-associated mortality and morbidity extends far beyond the acute phase of the disease. In earlier follow-up studies, as many as 30 % of the patients died during a follow-up period of up to 3 years, and up to 50 % of patients continued to complain of dyspnea and/or poor physical performance 6 months to 3 years after the index event. The most feared ‘late sequela’ of PE is chronic thromboembolic pulmonary hypertension (CTEPH), the true incidence of which remains obscure due to the large margin of error in the rates reported by mostly small, single-center studies. Moreover, the functional and hemodynamic changes corresponding to early, possibly reversible stages of CTEPH, have not been systematically investigated. The ongoing Follow-Up after acute pulmonary embolism (FOCUS) study will prospectively enroll and systematically follow, over a 2-year period and with a standardized comprehensive program of clinical, echocardiographic, functional and laboratory testing, a large multicenter prospective cohort of 1000 unselected patients (all-comers) with acute symptomatic PE. FOCUS will possess adequate power to provide answers to relevant remaining questions regarding the patients’ long-term morbidity and mortality, and the temporal pattern of post-PE abnormalities. It will hopefully provide evidence for future guideline recommendations regarding the selection of patients for long-term follow-up after PE, the modalities which this follow-up should include, and the findings that should be interpreted as indicating progressive functional and hemodynamic post-PE impairment, or the development of CTEPH.
Similar content being viewed by others
Unanswered questions after the acute phase of PE: the need for a large multicenter cohort study
Acute pulmonary embolism (PE) is a frequent cause of death and serious disability [1]. In an epidemiological model derived from six European countries, the estimated number of fatalities related to venous thromboembolism (VTE) amounted to 370,000, or 12 % of all deaths [2]. The risk of an adverse outcome during the acute phase varies widely depending on the clinical severity and the presence of right ventricular dysfunction at presentation [3], with early (30-day) mortality rates ranging from as low as 0.5 % in hemodynamically stable, ‘low-risk’ patients, to over 20 % in those presenting with cardiogenic shock [4]. Moreover, and importantly, the risk to die or develop persistent serious disability extends far beyond the acute phase of PE [5]. In earlier follow-up studies, as many as 25 % of the patients did not survive the first year after diagnosis, with the majority of deaths being related to underlying conditions such as cancer or chronic heart disease [6–8]. Consistently with these data, a cohort study of 866 patients who were retrospectively identified as having suffered acute PE (unprovoked in 308 patients; provoked in 558 patients), reported that 30 % of the study population died over a median follow-up period of 3.3 years [9]. Of 259 recorded deaths in that study, 110 were due to malignancy, 67 to recurrent PE, 30 to other cardiovascular disease, and the remaining 52 to other causes; in comparison, only 8.7 % of patients without PE died during the same study period [9]. Of note, there are no recent large multicenter cohort studies to provide contemporary, prospective data on long-term survival rates after PE.
Understanding and, as the following step, predicting the clinical course of a patient after acute PE is a far more complex task than answering the ‘dead or alive’ question. The available data from the follow-up of retrospectively identified patients, or from prospective studies of relatively small single-center cohorts (reviewed in [10]), suggest that more than 50 % of patients continue to complain of dyspnea and/or poor physical performance 6 months to 3 years after the index event, and up to 75 % perceive their own health status as being worse than before the acute PE episode. If confirmed in adequately powered follow-up studies, these numbers could be translated into a substantial clinical impact and socio-economic burden imposed by PE over the long term, far beyond that already acknowledged for the acute phase of the disease [11, 12]. At the far end of the severity spectrum of ‘late PE sequelae’ is a life-threatening disease termed chronic thromboembolic pulmonary hypertension (CTEPH). This progressive vasculopathy is thought to result from incomplete resolution of single or recurrent pulmonary emboli arising from sites of venous thrombosis [1, 13]. Although the diagnosis and management of CTEPH have made progress in recent years [14, 15], its true incidence and temporal pattern of development following an episode of acute PE remain obscure. The large margin of error in reported incidence rates of CTEPH (between 0.1 and 9.1 % within the first 2 years after a symptomatic PE event), is most likely due to referral bias, absence of early symptoms, and the difficulty in differentiating acute PE from an episode superimposed on pre-existing CTEPH [13, 16]. Moreover, and importantly, its ‘prodromi’, i.e. the clinical, functional and hemodynamic changes corresponding to early, possibly reversible stages of developing CTEPH, have not been systematically investigated.
The ongoing follow-up after acute pulmonary embolism (FOCUS) study will prospectively enroll and systematically follow, over a 2-year period and with a standardized comprehensive program of clinical, echocardiographic, functional and laboratory testing, a large multicenter prospective cohort of unselected patients (all-comers) hospitalized for acute symptomatic PE. FOCUS has been designed to possess adequate power which will enable it to provide answers to the above relevant remaining questions regarding the patients’ long-term morbidity and mortality after PE. It should thus be able to provide evidence for future guideline recommendations on which patients should be selected for long-term follow-up after PE, and possibly for ‘CTEPH screening’, and which modalities this follow-up should include.
Study population and objectives of FOCUS
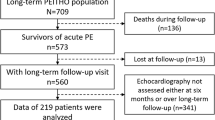
A total of 1000 consecutive patients with acute symptomatic, objectively diagnosed PE will be prospectively included in FOCUS on the basis of the eligibility criteria listed in Table 1. FOCUS explicitly aims to enroll ‘all-comers’ with PE, irrespective of the clinical severity, evidence of right ventricular dysfunction, or size or extent of pulmonary emboli. The primary objective is to determine the cumulative incidence of, (1) CTEPH, and (2) persisting or progressive functional and/or hemodynamic post-PE impairment (PPEI) over a 2-year follow-up period after an index episode of acute symptomatic PE. Secondary objectives are to assess, among others, overall and disease-specific long-term mortality, the incidence of major adverse cardiovascular events (such as acute myocardial infarction, stroke, or VTE recurrence), predictors as well as indicators of functional/hemodynamic impairment, fatal bleeding complications associated with long-term anticoagulant treatment for VTE, and the patients’ generic and disease-specific quality of life.
Patient outcomes
The complete list of primary, secondary, and safety outcomes of FOCUS is provided in Table 2. Confirmed diagnosis of CTEPH during the 2-year follow-up period is the first primary outcome. However, as the occurrence of CTEPH is expected to be low and the absolute number of events small, even with this large patient population, PPEI has been defined as a co-primary outcome. This approach, which is explained in the statistical analysis section below, has its rationale in the assumption that persisting or progressive functional and/or hemodynamic impairment after acute PE is an early indicator of, and in statistical terms a ‘necessary condition’ for, the subsequent development of CTEPH. PPEI was defined by the FOCUS steering committee based on (and extrapolated from), (1) previously proposed prognostic criteria for pulmonary hypertension [17], (2) practical guides to cardiopulmonary exercise testing for evaluation of pulmonary hypertension and chronic thromboembolic disease [18, 19], and (3) the risk assessment of pulmonary (arterial) hypertension at follow-up visits as recommended in the 2009 [20] and recently updated [14] guidelines of the European Society of Cardiology and the European Respiratory Society. A detailed overview of the assessment and classification of individual indicators of PPEI ((a) and (b) parameters) is provided in Table 3.
All outcomes will be adjudicated by an independent Clinical Events Committee (CEC).
Study design and flow
FOCUS is a prospective, multicenter, observational cohort study. The study protocol does not dictate any diagnostic or therapeutic interventions. On the other hand, the participating sites, which are high-volume centers with a long-standing experience in PE and pulmonary hypertension management, have harmonized their existing follow-up protocols and agreed that they will adhere to the follow-up schedule and workup presented in Table 4, which they consider as ‘best medical care’.
Detailed demographic and clinical data, diagnostic and therapeutic procedures, and outcome variables are recorded in an electronic case report form (eCRF). Regular follow-up visits are performed on discharge, and at 3, 12, and 24 months, as part of the best medical care standard at the participating centers.
Biobanking substudy: the FOCUS BioSeq project
A multicenter biobanking substudy ‘Biochemical and Genetic Biomarkers in Sequelae of Acute Pulmonary Embolism Study (FOCUS BioSeq)’ is being conducted within the FOCUS cohort. Currently, a number of biomarkers have a place in PE management; most of them, including d-dimers, cardiac troponins, and natriuretic peptides, are used in the differential diagnosis or risk stratification of acute PE [1]. On the other hand, a systematic biochemical and genetic characterization of PE survivors over the long term is missing in the literature. Thus, the primary objective of the FOCUS BioSeq substudy is to identify and evaluate molecular and genetic markers for late sequelae of acute PE. Further objectives are to evaluate novel and established biomarkers for cost effectiveness and suitability in long-term management strategies, and as predictors of the response to pharmacotherapy.
To achieve these goals, a decentralized and pseudonymized sample collection with centrifugal (standardized) preprocessing of plasma, serum and urine with short-term storage at −80 °C is being implemented in the participating study centers. For sample shipment to the central biobanking facility, pseudonyms and temperature control are maintained. On arrival, single sample aliquots are indexed for retrieval. Nucleic acids (DNA and RNA) are centrally isolated and quantity- and quality-controlled. Shipped, indexed and quality-controlled biomaterials are subsequently long-term stored in a centralized, 2-D barcoded and mirrored biobank at −80 °C. The following biomaterial specimens are collected in sufficient volumes for biomarker characterization and progression analysis in baseline and follow-up investigations: EDTA plasma, citrated plasma (3.2 %), serum, whole blood DNA and whole blood RNA (see Supplementary Table 1). Samples are collected and processed according to standard operating procedures (SOP). The FOCUS BioSeq biobanking procedures are illustrated in Fig. 1. Collection of biomaterial and optionally genetic examinations or exchange of biomaterial and data with collaboration partners are carried out after informed written consent has been provided by the study participant.
From the processed specimens, various biomarkers will be determined; a special focus will be put on biomarkers with known or expected relevance for the (pathophysiology of) circulation, hemostasis, inflammation, immunity, and kidney disease. High-throughput screening approaches will spotlight new potential biomarkers for sequelae of PE: Collected and purified DNA will be used for array-based genome-wide investigations aiming at discovering new genetic loci (further investigated by sequencing methods) which may predict the future clinical course of the disease or the response to drugs. Extracted RNA specimen like cellular mRNA, small non coding RNA or miRNA can be used for sequence probe based differential gene expression investigations. Epigenetic modifications on DNA samples or newly identified and known protein biomarkers at defined time points or in serial measurements from the follow-up assessment might be of prognostic relevance.
Sample size calculation and statistical analysis
For sample size calculation, we assumed that the annual incidence rate of CTEPH in unselected patients who have suffered an episode of acute symptomatic PE (either provoked or unprovoked) is at least five times higher than the very low rate of 0.16 per 100 patients per year reported in one of the largest cohorts published to date [25], i.e. 0.8 per 100 patients per year. We further assumed that overall death rates and case-mix (high risk versus intermediate-risk versus low risk PE) will be similar to that previous study [25], and that there will be a ≤5 % loss-to-follow-up per year in addition to the administrative censoring and deaths. Following these assumptions, the cumulative incidence of CTEPH at 2 years is expected to amount to 1.3 %. In this case, our simulations indicate that a study population of 1000 patients will provide roughly 90 % power to reject the H0 hypothesis that the cumulative incidence of CTEPH at 2-year follow-up is 0.27 % (the cumulative incidence corresponding to the rate reported in [25]).
For a subgroup with an expected size of about 500 patients with unprovoked PE, i.e. an index PE event in the absence of reversible predisposing factors, an exploratory analysis will be performed. In this analysis, a power of more than 80 % can still be achieved to reject the H0 hypothesis that the cumulative incidence of CTEPH at 2-year follow-up is 0.27 %, if the hazard in the subgroup is at least six times higher than that previously reported for the unselected PE population [9, 25].
All enrolled patients will be included in the statistical analysis. Hierarchical testing will be used for the two co-primary outcomes, CTEPH and PPEI, assuming that all patients will present with PPEI before or at the time of the diagnosis of CTEPH. Therefore, we will first test whether the cumulative incidence of PPEI is significantly higher than 0.27 % at 2 years, and, if this is the case, whether the cumulative incidence for CTEPH also differs from the same threshold. The overall level of significance will be set to α = 0.05. Both tests will have a local power of approximately 90 %. Testing will be performed by checking whether the 95 % confidence interval of the 2-year Aalen-Johansen estimate of the cumulative incidence function (CIF) contains the value 0.27 %.
For the analysis of the primary outcome, it is assumed that CTEPH and PPEI can be considered independent of possible deaths during follow-up (e.g., mortality associated with cancer, myocardial infarction or stroke); if the cause of death is not independent from PPEI, the analysis for the primary outcome assumes that CTEPH or PPEI is always diagnosed before death. While these assumptions can reasonably be expected to hold true for PPEI, they might be less robust for CTEPH. Therefore, this latter point will be addressed by sensitivity analyses. The planned secondary and sensitivity analyses are listed in the Supplementary Material.
Ethical aspects and data handling
The FOCUS study has been approved by the independent ethics committee at each participating site; it is being carried out in accordance with all local legal and regulatory requirements. All subjects must provide written informed consent to participate in the study, and a separate informed consent form is obtained for blood sampling and biodata banking. The study has been registered in the German Clinical Trials registry (http://www.germanctr.de; identifier: DRKS00005939).
As already mentioned, the study protocol does not dictate any diagnostic or therapeutic interventions; patients enrolled in FOCUS are treated according to current guidelines [1]. All findings, including clinical and laboratory data, are documented in individual medical records and in the eCRF. During entry of pseudonymized data, integrity checks help to minimize entry failures; any missing data or inconsistencies are reported back to the respective site and clarified by the responsible investigator. Regular monitoring is performed by personal visits from clinical monitors.
In case of withdrawal of a subject, the reason is documented and the patient is asked to consent to visit the clinic or a telephone interview in order to obtain as complete data as possible. Civil registers will be accessed or the patient´s family physician contacted if a personal contact is not possible.
Innovative features and expected impact of FOCUS
Existing data suggest that incomplete thrombus resolution as well as persisting hemodynamic abnormalities and functional limitation over the long term occur quite frequently after acute PE. For example, between 30 and 52 % of the patients who survive the acute phase have been reported to have evidence of residual perfusion defects for at least 1 year after the acute event [26–29]; furthermore, the proportion of patients with some degree of persistent pulmonary hypertension after acute PE ranged between 40 and 69 % [30, 31]. However, these data are to be interpreted with caution, since the single-center cohort studies conducted thus far included rather small numbers of patients, the echocardiographic parameters used to assess the hemodynamic status post PE did not rigorously follow the standards proposed by scientific societies [32], and a correlation of ultrasound or thrombus imaging findings with the severity of the patients’ residual symptoms or the degree of functional limitation at follow-up could not be established [33]. These limitations unavoidably generate a high degree of uncertainty in estimating and predicting late outcomes after PE, more specifically the risk of developing CTEPH [5] as well as the entirety of persisting or progressive abnormalities belonging to the so-called ‘post-PE syndrome’ [10]. In this context, it also needs to be mentioned that some patients who have had PE may present with chronic symptomatic disease, which is morphologically indistinguishable from CTEPH but associated with normal pulmonary hemodynamics at rest (on echocardiography and right heart catheterization). Although these patients are also considered to have CTEPH and are managed accordingly, the pathophysiology of this ‘chronic thromboembolic pulmonary vascular disease’ remains obscure, and an exact definition and appropriate terminology is still lacking. In view of all these uncertainties, even the most recent guidelines are unable to provide clear recommendations on who, how often, and with what modalities should be followed after acute PE [1, 14].
In order to effectively address these clinically relevant issues, the FOCUS study is equipped, by its design, with the following innovative elements:
-
a multicenter patient cohort with the participation of high-volume sites, geographically distributed all over Germany;
-
participating sites which possess recognized experience in the management of diseases of the pulmonary circulation and the right ventricle, and all fulfill high standards of patient care;
-
although FOCUS is not an interventional study and thus does not, per se, dictate diagnostic or therapeutic measures, the participating sites have standardized and harmonized their existing follow-up programs to reflect the ‘best current medical care’ of patients having suffered acute PE;
-
prospective enrollment of unselected ‘all-comers’ with acute symptomatic PE and prospective collection of data during multiple visits at predefined intervals;
-
a state of the art biobanking program;
-
a relatively short recruitment period of approximately 2 years in order to exclude the ‘confounder’ of major changes in the diagnostic and therapeutic approach to acute PE or in the follow-up phase during the study;
-
contemporary management of acute PE and CTEPH, reflecting the increasing role of the novel, direct oral anticoagulants, and implementing up-to-date algorithms for diagnostic workup of suspected CTEPH and patient management upon confirmation of the disease;
-
adequate sample size calculated to permit a reliable assessment of CTEPH rates after PE; even more important, prospective definition, as a co-primary outcome, of ‘post-PE impairment’ based on combinations of clinical, functional, hemodynamic, and imaging abnormalities which may persist or develop after the index episode; thus, the patients’ clinical course after acute PE is no longer reduced to a ‘yes or no’ question for CTEPH, but will be considered as a continuum of late post-PE complications;
-
sufficient power to determine clinical, functional, echocardiographic and laboratory determinants of CTEPH and/or PPEI.
As of August 2016, a total of 602 patients have been included at 19 active sites across Germany with a dropout rate of <5 %. Enrollment of the last patient is expected by the fourth quarter of 2017. The results of FOCUS and FOCUS BioSeq are expected to have a significant impact on the long-term management of patients after acute PE.
References
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk NA, Zamorano JL, Zompatori M (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC)endorsed by the European Respiratory Society (ERS). Eur Heart J 35(43):3033–3073. doi:10.1093/eurheartj/ehu283
Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M (2007) Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 98(4):756–764
Hobohm L, Hellenkamp K, Hasenfuss G, Munzel T, Konstantinides S, Lankeit M (2016) Comparison of risk assessment strategies for not-high-risk pulmonary embolism. Eur Respir J 47(4):1170–1178. doi:10.1183/13993003.01605-2015
Becattini C, Agnelli G, Lankeit M, Masotti L, Pruszczyk P, Casazza F, Vanni S, Nitti C, Kamphuisen P, Vedovati MC, De Natale MG, Konstantinides S (2016) Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. doi:10.1183/13993003.00024-2016
Konstantinides SV, Barco S, Lankeit M, Meyer G (2016) Management of pulmonary embolism: an update. J Am Coll Cardiol 67(8):976–990. doi:10.1016/j.jacc.2015.11.061
Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P (2004) Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 350(22):2257–2264
Eichinger S, Weltermann A, Minar E, Stain M, Schonauer V, Schneider B, Kyrle PA (2004) Symptomatic pulmonary embolism and the risk of recurrent venous thromboembolism. Arch Intern Med 164(1):92–96
Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J Jr, Hobbins TE (1992) The clinical course of pulmonary embolism. N Engl J Med 326(19):1240–1245
Klok FA, Zondag W, van Kralingen KW, van Dijk AP, Tamsma JT, Heyning FH, Vliegen HW, Huisman MV (2010) Patient outcomes after acute pulmonary embolism. A pooled survival analysis of different adverse events. Am J Respir Crit Care Med 181(5):501–506
Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S (2014) The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev 28(6):221–226. doi:10.1016/j.blre.2014.07.003
Konstantinides SV (2016) Trends in incidence versus case fatality rates of pulmonary embolism: good news or bad news? Thromb Haemost 115(2):233–235. doi:10.1160/TH15-10-0832
Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE (2016) European Union-28: an annualised cost-of-illness model for venous thromboembolism. Thromb Haemost 115(4):800–808. doi:10.1160/TH15-08-0670
Lang IM, Pesavento R, Bonderman D, Yuan JX (2013) Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J 41(2):462–468. doi:10.1183/09031936.00049312
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk NA, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz CA, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo BL, Albert BJ, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol C, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Voller H, Luis ZJ (2016) 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37(1):67–119. doi:10.1093/eurheartj/ehv317
Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, Lang I, Madani MM, Ogino H, Pengo V, Mayer E (2013) Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 62(25 Suppl):D92–D99. doi:10.1016/j.jacc.2013.10.024
Guerin L, Couturaud F, Parent F, Revel MP, Gillaizeau F, Planquette B, Pontal D, Guegan M, Simonneau G, Meyer G, Sanchez O (2014) Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Haemost 112(3). doi:10.1160/TH13-07-0538
McLaughlin VV, McGoon MD (2006) Pulmonary arterial hypertension. Circulation 114(13):1417–1431. doi:10.1161/CIRCULATIONAHA.104.503540
Wensel R, Francis DP, Meyer FJ, Opitz CF, Bruch L, Halank M, Winkler J, Seyfarth HJ, Glaser S, Blumberg F, Obst A, Dandel M, Hetzer R, Ewert R (2013) Incremental prognostic value of cardiopulmonary exercise testing and resting haemodynamics in pulmonary arterial hypertension. Int J Cardiol 167(4):1193–1198. doi:10.1016/j.ijcard.2012.03.135
Held M, Kolb P, Grun M, Jany B, Hubner G, Grgic A, Holl R, Schaefers HJ, Wilkens H (2016) Functional characterization of patients with chronic thromboembolic disease. Respiration 91(6):503–509. doi:10.1159/000447247
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 34(6):1219–1263
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4):692–694. doi:10.1111/j.1538-7836.2005.01204.x
Held M, Grun M, Holl R, Hubner G, Kaiser R, Karl S, Kolb M, Schafers HJ, Wilkens H, Jany B (2014) Cardiopulmonary exercise testing to detect chronic thromboembolic pulmonary hypertension in patients with normal echocardiography. Respiration 87(5):379–387. doi:10.1159/000358565
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20(10):1727–1736. doi:10.1007/s11136-011-9903-x
Klok FA, Cohn DM, Middeldorp S, Scharloo M, Buller HR, van Kralingen KW, Kaptein AA, Huisman MV (2010) Quality of life after pulmonary embolism: validation of the PEmb-QoL Questionnaire. J Thromb Haemost 8(3):523–532. doi:10.1111/j.1538-7836.2009.03726.x
Klok FA, van Kralingen KW, van Dijk AP, Heyning FH, Vliegen HW, Huisman MV (2010) Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 95(6):970–975. doi:10.3324/haematol.2009.018960
Cosmi B, Nijkeuter M, Valentino M, Huisman MV, Barozzi L, Palareti G (2011) Residual emboli on lung perfusion scan or multidetector computed tomography after a first episode of acute pulmonary embolism. Intern Emerg Med 6(6):521–528
Sanchez O, Helley D, Couchon S, Roux A, Delaval A, Trinquart L, Collignon MA, Fischer AM, Meyer G (2010) Perfusion defects after pulmonary embolism: risk factors and clinical significance. J Thromb Haemost 8(6):1248–1255
Nijkeuter M, Hovens MM, Davidson BL, Huisman MV (2006) Resolution of thromboemboli in patients with acute pulmonary embolism: a systematic review. Chest 129(1):192–197
Miniati M, Monti S, Bottai M, Scoscia E, Bauleo C, Tonelli L, Dainelli A, Giuntini C (2006) Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine (Baltimore) 85(5):253–262. doi:10.1097/01.md.0000236952.87590.c8
Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA (2009) Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 136(5):1202–1210
Stevinson BG, Hernandez-Nino J, Rose G, Kline JA (2007) Echocardiographic and functional cardiopulmonary problems 6 months after first-time pulmonary embolism in previously healthy patients. Eur Heart J 28(20):2517–2524
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713. doi:10.1016/j.echo.2010.05.010
Chung T, Emmett L, Mansberg R, Peters M, Kritharides L (2007) Natural history of right ventricular dysfunction after acute pulmonary embolism. J Am Soc Echocardiogr 20(7):885–894. doi:10.1016/j.echo.2006.12.005
Acknowledgments
We are grateful to Kurt Quitzau and Sabrina Rump for their contribution to the design, preparation and coordination of the FOCUS study and the FOCUS BioSeq biobanking project.
Funding
FOCUS is an independent, investigator-initiated study. The study has an academic sponsor (University Medical Center of the Johannes Gutenberg University, Mainz, Germany). The investigators are responsible for the design, conduct, and analysis of FOCUS. The work of Stavros Konstantinides, Stefano Barco, Mareike Lankeit, Nadine Heydenreich, Antonio Pinto, Bianca Zäpf and Philipp Wild is supported by the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503). In addition, the sponsor has obtained grants from Bayer Vital GmbH and Bayer Pharma AG.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Konstantinides, S.V., Barco, S., Rosenkranz, S. et al. Late outcomes after acute pulmonary embolism: rationale and design of FOCUS, a prospective observational multicenter cohort study. J Thromb Thrombolysis 42, 600–609 (2016). https://doi.org/10.1007/s11239-016-1415-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-016-1415-7