Abstract
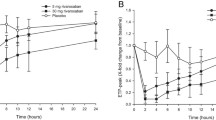
We performed detailed pharmacokinetic and pharmacodynamic modeling of REG1, an anticoagulation system composed of the direct factor IXa (FIXa) inhibitor pegnivacogin (RB006) and its matched active control agent anivamersen (RB007), with a focus on level of target inhibition to translate phase 1 results to phase 2 dose selection. We modeled early-phase clinical data relating weight-adjusted pegnivacogin dose and plasma concentration to prolongation of the activated partial thromboplastin time (aPTT). Using an in vitro calibration curve, percent FIXa inhibition was determined and related to aPTT prolongation and pegnivacogin dose and concentration. Similar methods were applied to relate anivamersen dose and level of reversal of pegnivacogin anticoagulation. Combined early-phase data suggested that ≥0.75 mg/kg pegnivacogin was associated with >99% inhibition of FIX activity and prolongation of plasma aPTT values ≈2.5 times above baseline, leading to selection of a 1 mg/kg dose for a phase 2a elective percutaneous coronary intervention study to achieve a high intensity of anticoagulation and minimize intersubject variability. Phase 2 validated our predictions, demonstrating 1 mg/kg pegnivacogin yielded plasma concentrations ≈25 μg/ml and >99% inhibition of FIX activity. The relationship between the anivamersen to pegnivacogin dose ratio and degree of pegnivacogin reversal was also validated. Our approach decreased the need for extensive dose–response studies, reducing the duration, complexity and cost of clinical development. The 1 mg/kg pegnivacogin dose and a range of anivamersen dose ratios are being tested in the phase 2b RADAR study (NCT00932100).





Similar content being viewed by others
References
Dyke CK, Steinhubl SR, Kleiman NS, Cannon RO, Aberle LG, Lin M, Myles SK, Melloni C, Harrington RA, Alexander JH, Becker RC, Rusconi CP (2006) First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation 114(23):2490–2497
Howard EL, Becker KCD, Rusconi CP, Becker RC (2007) Factor IXa inhibitors as novel anticoagulants. Arterioscler Thromb Vasc Biol 27(4):722–727
Rusconi CP, Scardino E, Layzer J, Pitoc GA, Ortel TL, Monroe D, Sullenger BA (2002) RNA aptamers as reversible antagonists of coagulation factor IXa. Nature 419(6902):90–94
Chan MY, Cohen MG, Dyke CK, Myles SK, Aberle LG, Lin M, Walder J, Steinhubl SR, Gilchrist IC, Kleiman NS, Vorchheimer DA, Chronos N, Melloni C, Alexander JH, Harrington RA, Tonkens RM, Becker RC, Rusconi CP (2008) Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation 117(22):2865–2874
Chan MY, Rusconi CP, Alexander JH, Tonkens RM, Harrington RA, Becker RC (2008) A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor IXa inhibitor. J Thromb Haemost 6(5):789–796
Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Myles SK, Aberle LG, Greenbaum AB, Fry E, Chan MY, Tonkens RM, Zelenkofske S, Alexander JH, Harrington RA, Rusconi CP, Becker RC (2010) First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous intervention. Circulation 122(6):614–622
Gopinath SCB, Shikamoto Y, Mizuno H, Kumar PKR (2006) A potent anti-coagulant RNA aptamer inhibits blood coagulation by specficially blocking the extrinsic clotting pathway. Thromb Haemost 95(5):767–771
Povsic TJ, Cohen MG, Mehran R, Buller CE, Bode C, Cornel JH, Kasprzak JD, Montalescot G, Joseph D, Wargin WA, Rusconi CP, Zelenkofske SL, Becker RC, Alexander JH (2011) A randomized, partially blinded, multicenter, active-controlled, dose-ranging study assessing the safety, efficacy, and pharmacodynamics of the REG1 anticoagulation system in patients with acute coronary syndromes: design and rationale of the RADAR phase IIb trial. Am Heart J 161(2):261–268
Acknowledgments
We would like to thank the CLIN101, 102, 103, and 210 clinical site investigators and research team personnel who diligently executed the study protocols, provided important feedback for future study design, and produced high quality data to support the continued development of the REG1 System. We also thank Elizabeth Cook for exceptional editorial input and assistance.
Disclosure
Dr. Rusconi is a founder, employee, and stockholder in REGADO Biosciences. Dr. Zelenkofske is an employee and stockholder in REGADO Biosciences. Dr. Wargin is a consultant for REGADO Biosciences. Drs. Becker, Povsic, Harrington, and Alexander are employed by the Duke Clinical Research Institute which has received research funding from REGADO Biosciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Povsic, T.J., Cohen, M.G., Chan, M.Y. et al. Dose Selection for a Direct and Selective Factor IXa Inhibitor and its Complementary Reversal Agent: Translating Pharmacokinetic and Pharmacodynamic Properties of the REG1 System to Clinical Trial Design. J Thromb Thrombolysis 32, 21–31 (2011). https://doi.org/10.1007/s11239-011-0588-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-011-0588-3