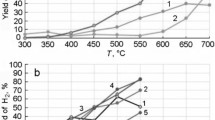
The acidity and basicity of MFe2O4 (M(II) = Fe, Mg, Mn, Zn) ferrites of the spinel structure have been characterized by temperature-programmed desorption of NH3 and CO2. It is shown that both medium-strength basic and acid sites play an important role in the process of acetone obtaining from ethanol over ferrites. The selectivity of ethanol conversion to acetone depends both on the acid–base properties of the surface and on the ability of the surface oxygen of ferrite to participate in the intermediate redox stages of formation and steam reforming of acetone.


Similar content being viewed by others
References
A. F. F. De Lima, C. R. Moreira, O. C. Alves, et al., Appl. Catal. A., 611, 117949 (2021).
C. P. Rodrigues, P. C. Zonetti, and L. G. Appel, Chem. Cent. J., 11, 30 (2017).
L. R. Silva-Calpa, P. C. Zonetti, D. C. Oliveira, et al., Catal. Today, 289, 264-272 (2017).
X. Li, A. Kant, Y. He, et al., Catal. Today, 276, 62-77 (2016).
V. Zacharopoulou and A. A. Lemonidou, Catalysts, 8, 2-19 (2018).
R. Huang, V. Fung, Z. Wu, and D. Jiang, Catal. Today, 350, 19-24 (2019).
F. Hayashi, M. Tanaka, D. Lin, and M. Iwamoto, J. Catal., 316, 112-120 (2014).
M. Iwamoto, Catal. Today, 242, 243-248 (2015).
Y. Pyatnitsky, L. Dolgikh, L. Senchylo, et al., Theor. Exp. Chem., 55, 50-55 (2019).
Y. Pyatnitsky, L. Dolgikh, L. Senchylo, et al., Chem. Pap., 75, 5773-5779 (2021).
L. Y. Dolgikh, I. L. Stolyarchuk, L. A. Staraya, et al., Theor. Exp. Chem., 54, 349-357 (2018).
T. Nakajima, H. Nameta, S. Mishima, et al., J. Mater. Chem., 4, 853-858 (1994).
J. I. Di Cosimo, V. K. Diez, M. Xu, et al., J. Catal., 178, 499-510 (1998).
H. Song, L. Zhang, and U. S. Ozkan, Top. Catal., 55, 1324-1331 (2012).
J. Sun, K. Zhu, F. Gao, et al., J. Am. Chem. Soc., 133, 11096-11099 (2011).
I. L. Stolyarchuk, L. Yu. Dolgykh, I. V. Vasylenko, et al., Theor. Exp. Chem., 52, 246-251 (2016).
A. Auroux and A. Gervasini, Phys.Chem., 94, 6371-6379 (1990).
M. B. Jensen, L. G. M. Pettersson, O. Swang, et al., J. Phys. Chem. B., 109, 16774-16781 (2005).
V. K. Diez, C. R. Apestegua, and J. I. Di Cosmo, J. Catal., 240, 235-244 (2006).
R. S. Murthy, P. Patnaik, P. Sidheswaran, et al., J. Catal., 109, 298-302 (1988).
K. Inui, T. Kurabayashi, and S. Sato, J. Catal., 212, 207-215 (2002).
T. Nishiguchi, T. Matsumoto, H. Kanai, et al., Appl. Catal. A., 279, 73-77 (2005).
T. Nakajima, K. Tanabe, T. Yamaguchi, et al., Appl. Catal., 52, 237-248 (1989).
J. Bussi, S. Parodi, B. Irigaray, et al., Appl. Catal. A., 172, 117-129 (1998).
H. Hattori, Chem. Rev., 95, 537-558 (1995).
F. Hayashi, M. Tanaka, D. Lin, et al., J. Catal., 316, 112-120 (2014).
G. G. Gonzalez, P. C. Zonetti, E. B. Silveira, et al., J. Catal., 380, 343-351 (2019).
K. Takeshita, S. Nakamura, and K. Kawamoto, Bull. Chem. Soc. Jpn., 51, 2622-2627 (1978).
L. V. Mattos, G. Jacobs, B. H. Davis, et al., Chem. Rev., 112, 4094-4123 (2012).
J. I. Di Cosmo, C. R. Apesteguia, M. J. L. Gines, et al., J. Catal., 190, 261-275 (2000).
M. V. Ganduglia-Pirovano, Catal. Today, 253, 20-32 (2015).
J. E. Rorrer, F. D. Toste, and A. T. Bell, ACS Catal., 9, 10588-10604 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 58, No. 4, pp. 259-264, July-August, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dolgikh, L.Y., Stolyarchuk, I.L., Stara, L.O. et al. Influence of Acid–Base Properties of MFe2O4 Ferrites (M(II) = Fe, Mg, Mn, Zn) on Their Selectivity in the Conversion of Ethanol to Acetone. Theor Exp Chem 58, 290–296 (2022). https://doi.org/10.1007/s11237-022-09746-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-022-09746-1