Abstract
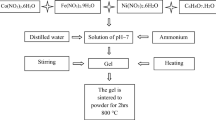
A noticeable improvement in the structural properties of Mn–Zn ferrite obtained by the solution combustion method was observed when the gel was additionally dried before self-combustion. The catalytic activity of thus prepared nanosized Mn–Zn ferrite has been also studied. The powders after combustion process were analyzed by X-ray diffraction and it was found that they possess pure spinel phase. The ferrite particles were agglomerated wherein powders obtained from the modified solution combustion method (gel additionally dried) characterized sponge-like structure and developed specific surface area. This ferrite material exhibits a significantly higher total acidity and it is reduced in the lower temperature. As a ferrite catalyst, prepared by the modified solution combustion method, possesses both acid and base centers, which allows to proceed the bimolecular ketonization reaction. The yield and selectivity of ketone increase with temperature with a maximum at 440 °C. Taking into account the obtained yield and selectivity results in the butan-1-ol conversion the Mn0.6Zn0.4Fe2O4 ferrite prepared in low temperature can be used as potential dehydrogenation catalyst.








Similar content being viewed by others
References
Goldman A (2006) Modern ferrite technology, 2nd edn. Springer, Pittsburgh
Hessien MM, Rashad MM, El-Barawy K, Ibrahim IA (2008) Influence of manganese substitution and annealing temperature on the formation, microstructure and magnetic properties of Mn–Zn ferrites. J Magn Magn Mater 320:1615–1621
Rath C, Mishra NC, Anand S, Das RP, Sahu KK, Upadhyay C, Verma HC (2000) Appearance of superparamagnetism on heating nanosize Mn0.65Zn0.35Fe2O4. App Phys Lett 76:475–477
Cao Y, Ren J, Li JB, Liu Y (2011) Thermo-responsive Mn–Zn ferrite/poly(N,N′-isopropyl acrylamide-co-N-hydroxymethylacrylamide) core/shell nanocomposites for drug-delivery systems. J Biomater Sci Polym Ed 22:1473–1486
Sharifi I, Shokrollahi H, Amiri S (2012) Ferrite-based magnetic nanofluids used in hyperthermia applications. J Magn Magn Mater 324:903–915
Guével X, Prinz EM, Müller R, Hempelmann R, Schneider M (2012) Synthesis and characterization of superparamagnetic nanoparticles coated with fluorescent gold nanoclusters. J Nanopart Res 14:1–10
Shah SA, Majeed A, Rashid K, Awan SU (2013) PEG-coated folic acid-modified superparamagnetic MnFe2O4 nanoparticles for hyperthermia therapy and drug delivery. Mater Chem Phys 138:703–708
Hou XY, Feng J, Liu XH, Ren YM, Fan ZJ, Zhang ML (2011) Magnetic and high rate adsorption properties of porous Mn1−xZnxFe2O4 (0 ≤ x ≤ 0.8) adsorbents. J Coll Interf 353:524–529
Laohhasurayotin K, Pookboonmee S, Viboonratanasri D, Kangwansupamonkon W (2012) Preparation of magnetic photocatalyst nanoparticles TiO2/SiO2/Mn–Zn ferrite and its photocatalytic activity influenced by silica interlayer. Mater Res Bull 47:1500–1507
Klimkiewicz R, Przybylski K, Baran J, Miśta W (2009) Mg–Zn and Mn-Zn ferrites derived from coil core materials as new precursors for catalysts of primary alcohols. Ind Eng Chem Res 48:6291–6295
Klimkiewicz R, Grabowska H, Miśta W, Przybylski K (2012) Mg–Zn and Mn–Zn ferrites derived from coil core materials as new phenol methylation catalysts. Ind Eng Chem Res 51:2205–2213
Kadu AV, Jagtap SV, Chaudhari GN (2009) Studies on the preparation and ethanol gas sensing properties of spinel Zn0.6Mn0.4Fe2O4 nanomaterials. Curr Appl Phys 9:1246–1251
Ramankutty CG, Sugunan S, Thomas B (2002) Study of cyclohexanol decomposition reaction over the ferrospinels, A1-xCuxFe2O4 (A = Ni or Co and x = 0, 0.3, 0.5, 0.7 and 1), prepared by ‘soft’ chemical methods. J Mol Catal A-Chem 187:105–117
Jacobs JP, Maltha A, Reitntjes JGS, Drimal J, Ponec V, Brongersma HH (1994) The surface of catalytically active spinels. J Catal 147:294–300
Zahraei M, Monshi A, Morales DP, Shahbazi-Gahrouei D, Amirnasr M, Behdadfar B (2015) Hydrothermal synthesis of fine stabilized superparamagnetic nanoparticles of Zn2+ substituted manganese ferrite. J Magn Magn Mater 393:429–436
Phong PT, Nam PH, Manh DH, Tung DK, Lee I-J, Phuc NX (2015) Studies of the magnetic properties and specific absorption of Mn0.3Zn0.7Fe2O4 nanoparticles. J Electron Mater 44:287–294
Kumar S, Shinde TJ, Vasambekar PN (2015) Study of conduction phenomena in indium substituted Mn–Zn nano-ferrites. J Magn Magn Mater 379:179–185
Kareem SH, Ati AA, Shamsuddin M, Lee SL (2015) Nanostructural, morphological and magnetic studies of PEG/Mn(1−x)Zn(x)Fe2O4 nanoparticles synthesized by co-precipitation. Ceram Int 41:11702–11709
Jagadeesha Angadi V, Rudraswamy B, Sadhana K, Murth SR, Praveena K (2016) Effect of Sm3+ Gd3+ on structural, electrical and magnetic properties of Mn–Zn ferrites synthesized via combustion route. J Alloys Compd 656:5–12
Peng Y, Chen L, Ren H, Li L, Yi J, Xia Q (2016) Structure and properties of MnZn ferrite nanoparticles synthesized via sol–gel autocombustion method. J Mater Sci 27:587–591
Masthoff I-C, Gutsche A, Nirschl H, Garnweitner G (2015) Oriented attachment of ultrasmall Mn(1−x)ZnxFe2O4 nanoparticles during the non-aqueous sol–gel synthesis. Cryst Eng Comm 17:2464–2470
Beji Z, Sun M, Smiri L, Herbst F, Mangeney C, Ammar S (2015) Polyol synthesis of nonstoichiometric Mn–Zn ferrite nanocrystals: structural/microstructural characterization and catalytic application. RSC Adv 5:65010–65022
Abbas M, Torati SR, Rao BP, Abdel-Hamed MO, Kim C (2015) Size controlled sonochemical synthesis of highly crystalline superparamagnetic Mn–Zn ferrite nanoparticles in aqueous medium. J Alloys Compd 64:774–782
Patil KC, Aruna ST, Ekambaram S (1997) Combustion synthesis. Curr Opin Solid St M 2:158–165
Mukasyan AS, Dinka P (2007) Novel approaches for solution combustion synthesis of nano-materials. Int J Self Propag High Temp Synth 16:23–35
Deshpande K, Mukasyan A, Varma A (2004) Direct synthesis of iron oxide nanopowders by the combustion approach: reaction mechanism and properties chemistry of materials. Chem Mater 16:4896–4904
Yang F, Wei JJ, Liu W, Guo JX, Yang YZ (2014) Copper doped ceria nanospheres: surface defects promoted catalytic activity and a versatile approach. J Mater Chem A 2:5662–5667
Moroz EM, Zyuzin DA, Tregubenko VY, Udras IE, Belyi AS, Likholobov VA (2013) Effect of structural defects in alumina supports on the formation and catalytic properties of the active component of reforming catalysts. Reac Kinet Mech Cat 110:459–470
Lahiri P, Sengupta SK (1991) Spinel ferrite as catalysts: a study on catalytic effect of coprepcipitation ferrites on hydrogen peroxide decomposition. Can J Chem 69:33–36
Sungnan S, John D, Renuka NK, Varghese M, Sreekumar K, Ramankutty CG (1999) Electron donor properties and catalytic activity of manganese ferrospinels. React Kinet Catal Lett 66:39–45
Vijayaraj M, Gopinath CS (2006) On the “Active spacer and stabilizer” role of Zn in Cu1-xZnxFe2O4 in the selective mono-N-methylation of aniline: XPS and catalysis study. J Catal 241:83–95
Winiarska K, Szczygieł I, Klimkiewicz R (2013) Manganese–zinc ferrite synthesis by the sol gel autocombustion method. Effect of the precursor on the ferrite’s catalytic properties. Ind Eng Chem Res 52:353–361
Feng Q, Ma XH, Yan QZ, Ge CC (2009) Preparation of soft-agglomerated nano-sized ceramic powders by sol–gel combustion process. Mater Sci Eng B 162:53–58
Szczygieł I, Winiarska K, Bieńko A, Suracka K, Gaworska-Koniarek D (2014) The effect of the sol–gel autocombustion synthesis conditions on the Mn–Zn ferrite magnetic properties. J Alloys Comp 604:1–7
Klug HP, Alexander LE (1974) X-ray diffraction procedures for polycrystalline and amorphous materials. Willey, New York
Patil RP, Delekar SD, Mane DR, Hankare PP (2013) Synthesis, structural and magnetic properties of different metal ion substituted nanocrystalline zinc ferrite. Results Phys 3:129–133
Angermann A, Töpfer J (2011) Synthesis of nanocrystalline Mn–Zn ferrite powders through thermolysis of mixed oxalates. Ceram Int 37:995–1002
Yue Z, Guo W, Zhou J, Gui Z, Li L (2004) Synthesis of nanocrystilline ferrites by sol–gel combustion process: the influence of pH value of solution. J Magn Magn Mater 270:216–223
Giri J, Srihasha T, Asthana S, Rao TKG, Nigam AK, Bahadur D (2005) Synthesis of capped nanosized Mn1−xZnxFe2O4 (0 ≤ x ≤ 0.8) by microwave refluxing for biomedical applications. J Magn Magn Mater 293:55–61
Li J, Wu YS, Pan YB, Liu WB, Zhu Y, Guo JK (2008) Agglomeration of alpha-Al2O3 powders prepared by gel combustion. Ceram Int 34:1539–1542
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure and Appl Chem 57:603–619
Kumar KNP, Kumar J, Keizer K (1994) Effect of peptization on densification and phasetransformation behavior of sol–gel-derived nanostructured titania. J Am Ceram Soc 77:1396–1400
Ramankutty CG, Sugunan S (2001) Surface properties and catalytic activity of ferrospinels of nickel, cobalt and copper, prepared by soft chemical methods. Appl Catal A 218:39–51
Benrabaa R, Boukhlouf H, Löfberg A, Rubbens A, Vannier RN, Bordes-Richard E, Barama A (2012) Nickel ferrite spinel as catalyst precursor in the dry reforming of methane: synthesis, characterization and catalytic properties. J Nat Gas Chem 21:595–604
Khan A, Chen P, Boolchand P, Smirniotis PG (2008) Modified nano-crystalline ferrites for high-temperature WGS membrane reactor applications. J Catal 253:91–104
Zhang LF, Wu YX (2013) Sol–gel synthesized magnetic spinel ferrite nanoparticles as novel catalyst for oxidative degradation of methyl orange. J Nanomater 2013: 1–6. ID640940
Wimmers OJ, Arnoldy P, Moulijn JA (1986) Determination of the reduction mechanism by temperature-programmed reduction: application to small Fe2O3 particles. J Phys Chem 90:1331–1337
Corson BB, Ipatieff VN (1939) Simultaneous dehydrogenation-hydrogenation of cyclohexene in the presence of nickel. J Am Chem Soc 61:1056–1057
Michorczyk P, Ogonowski J (2006) Simultaneous propane dehydrogenation and CO2 hydrogenation over CrOx/SiO2 catalyst. React Kinet Catal Lett 87:177–183
Shen WQ, Tompsett GA, Xing R, Conner WC Jr, Huber GW (2012) Vapor phase butanal self-condensation over unsupported and supported alkaline earth metal oxides. J Catal 286:248–259
Phung TK, Casazza AA, Aliakbarian B, Finocchio E, Perego P, Busca G (2013) Catalytic conversion of ethyl acetate and acetic acid on alumina as models of vegetable oils conversion to biofuels. Chem Eng J 215–216:838–848
Klimkiewicz R, Grabowska H, Syper L (2003) Vapor-phase conversion of esters into ketones in the presence of an Sn, Ce, and Rh containing oxide catalyst. Kinet Catal 44:283–286
Ordomsky VV, Sushkevich VL, Ivanova II (2010) Study of acetaldehyde condensation chemistry over magnesia and zirconia supported on silica. J Mol Catal 333:85–93
Teterycz H, Klimkiewicz R, Łaniecki M (2003) The role of Lewis acidic centers in stabilized zirconium dioxide. Appl Catal A 249:313–326
Acknowledgements
The authors are grateful to Professor Leszek Kępiński from Institute of Low Temperature and Structural Research (Polish Academy of Science) for the TEM investigations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winiarska, K., Klimkiewicz, R., Winiarski, J. et al. Mn0.6Zn0.4Fe2O4 ferrites prepared by the modified combustion method as the catalyst for butan-1-ol dehydrogenation. Reac Kinet Mech Cat 120, 261–278 (2017). https://doi.org/10.1007/s11144-016-1095-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-016-1095-5