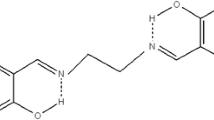
A study was carried out on the kinetics of the reaction of 1-chloro-2,3-epoxypropane (epichlorohydrin, ECH) with aliphatic carboxylic acids in the presence of (3-chloro-2-hydroxypropyl)trimethylammonium (CHPTMA) chloride obtained by trimethylammonium chloride and ECH. We compared the catalytic activity of CHPTMA chloride, trialkylamines, and tetraalkylammonium halides and evaluated the effect of their structure. An ab initio quantum-chemical calculation was performed to obtain a detailed analysis of the state of the catalysts in this system in order to understand the peculiarities of catalytic activity of these bases.
Similar content being viewed by others
References
L. I. Kas'yan, A. O. Kas'yan, S. I. Okovityi, et al., Reactivity of Acyclic Epoxide Compounds [in Russian], Izd. Dnepropetrovsk. Univ., Dnepropetrovsk, Ukraine (2003).
A. F. Maslyuk, G. K. Bereznitskii, V. K. Grishchenko, et al., Ukr. Khim. Zh., 50, No. 1, 92–97 (1984).
A. M. Paken, Epoxide Compounds and Epoxy Resins [in Russian], Goskhimizdat, Leningrad (1962).
R. N. Armstrong and C. S. Cassidy, Drug Metabol. Rev., 32, Nos. 3/4, 327–338 (2000).
F. P. Guengerich and W. W. Johnson, Drug Metabol. Rev., 31, No. 1, 141–158 (1999).
A. K. Gus'kov, Yuy Shushen, M. G. Makarov, et al., Kinet. Katal., 35, No. 6, 873–877 (1994).
E. N. Shved, V. V. Usachev, and E. I. Kozorezova, Ukr. Khim. Zh., 73, No. 12, 113–117 (2007).
Yu. N. Bespal'ko, E. N. Shved, and T. A. Chepurnaya, Ukr. Khim. Zh., 74, No. 2, 120–124 (2008).
B. P. Nikol'skii (ed.), Chemist's Handbook [in Russian], Khimiya, Leningrad (1971).
H. K. Reimschuessel and M. A. Kocur, “Process for the preparation of anhydrous N-(3-chloro-2-hydroxypropyl) trialkylammonium salts,” US Patent No. 4,594,452, IPC C 08 G 73/18; C 08 G73/00; (IPC-1-7) C 07 C 91/26, Publ. October 6, 1986.
V. V. Usachev, E. N. Shved, E. I. Kozorezova, et al., Ukr. Khim. Zh., 72, No. 4, 108–112 (2006).
A. B. Hoefelmeyer and C. K. Hancock, J. Am. Chem. Soc., 77, 4726–4748 (1955).
A. S. Dneprovskii and T. I. Temnikova, Theoretical Fundamentals of Organic Chemistry [in Russian], Khimiya, Leningrad (1991).
A. A. Granovsky, PC GAMESS version 7.0. http://classic.chem.msu.su/gran/gamess/index.html.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental'naya Khimiya, Vol. 44, No. 5, pp. 292–297, September–October, 2008.
Rights and permissions
About this article
Cite this article
Bespal’ko, Y.N., Shved, E.N. & Oleinik, N.M. Peculiarities of catalytic behavior of bases in the reaction of aliphatic carboxylic acids with epoxide. Theor Exp Chem 44, 300–306 (2008). https://doi.org/10.1007/s11237-008-9041-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-008-9041-x