Abstract
Species of genus Polymorphus Lühe, 1911 (Polymorphidae) are acanthocephalans found in fish-eating birds and waterfowl. Although found in many parts of the world, including Australia, no records exist from New Zealand. Because of the largely aquatic intermediate host, Polymorphus species are rarely found in terrestrial birds of prey. During a study of the helminths of the Australasian harrier Circus approximans Peale specimens of Polymorphus were recovered that were found to be new to science. Polymorphus circi n. sp. is formally described and genetic sequence data presented. Specimens were distinguished from all other species by a combination of characters, including their proboscis hook arrangement (20–22 rows of 11–13 hooks), as well as absence of sexual dimorphism, trunk size, proboscis shape and egg size. These acanthocephalans were found in birds from areas with the potential to support freshwater, brackish or marine amphipods, but as yet the actual intermediate hosts are unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Polymorphus Lühe, 1911 (Acanthocephala: Polymorphida: Polymorphidae) is a widespread group of parasite species that infect the gastrointestinal tract of fish-eating birds and waterfowl, and use amphipods as intermediate hosts (Crompton & Nickol 1985; Amin 1992). Species of Polymorphus have been recorded from Europe, North and South America, Russia and Pakistan, and one of the 36 known species is found in Australia (Amin 1992; Johnston & Edmonds 1948). Apart from an unnamed larval stage in a hedgehog (Smales et al. 2010), there are no records from New Zealand. The taxonomic history of genus Polymorphus is complicated, with various other genera having been treated as synonyms (Profilicollis Meyer, 1931, Falsifilicollis Webster, 1948, Parafilicollis Petrochenko, 1956, Subfilicollis Hoklova, 1967, Subcorynosoma Hoklova, 1967, Arhythmorhynchus Lühe, 1911, Hexaglandula Petrochenko, 1950) or as subgenera (Profilicollis, Hexaglandula) in earlier taxonomic treatments (Yamaguti 1963; Amin 1992). The advent of phylogenetics has enabled some clarity, confirming the generic identity of Profillicollis, Arhythmorhynchus and Hexaglandula (García-Varela et al. 2011, 2013; García-Varela & Pérez-Ponce de León, 2008). However, all of these studies produce phylogenetic hypotheses in which available sequences of Polymorphus are polyphyletic, indicating that species need to be re-examined and reclassified using morphological and ecological data, and a broader molecular range.
The Australasian harrier Circus approximans Peale (Accipitriformes: Accipitridae), also known as swamp harrier, harrier hawk or kāhu, is native to Australia, New Zealand and some islands in the South Pacific (Debus & Kirwan 2020). It is an opportunistic hunter of live prey such as small birds, mammals and invertebrates, and also a scavenger, with carrion making up a major component of the diet (Baker-Gabb 1981). In New Zealand, harriers hunt mainly in open habitats, and the population has benefitted from widespread deforestation for agriculture. Along with the ready availability of road-kill carcasses, this has seen the harrier rise to healthy population numbers (Eakle 2008). Its conservation status is Not Threatened (Robertson et al. 2021), but the bird is considered a taonga (“treasured”) species by Māori, and is partially protected by law (Wildlife (Australasian Harrier) Notice 2012). Many harriers are themselves victims of roadkill or injury (Sadleir & Linklater 2016), meaning considerable numbers are available for study. In Australasian harriers, only one acanthocephalan has been reported in Australia (Centrorhynchus asturinus (Johnston, 1913) in Smales 2003), and no records exist for the New Zealand population.
The opportunity to examine a large number of deceased harriers from the southern half of South Island since 2017 has allowed the authors to conduct a survey of all helminth parasites found, and what follows is a description of a polymorphid acanthocephalan found in some birds, which was found to be new to science. We provide 28S rDNA sequence for future comparison. A description of a new species of cestode and a report on other helminths recovered from the NZ harriers, including a new species of nematode, will be published elsewhere.
Materials and methods
Harrier collection and processing
A total of 65 harriers was examined: 41 from Otago, donated by the Dunedin Wildlife Hospital between 2017 and 2022; 19 from Canterbury, donated by The New Zealand Raptor Trust in 2022/3, and five collected as roadkill by the first author. Birds were frozen for storage, defrosted prior to dissection, and helminths were collected and preserved in 70% ethanol for whole-mount, 96% ethanol for genetic analyses and 4% buffered formalin for SEM imaging.
Morphological data
Acanthocephalan specimens were cleared and mounted temporarily in beechwood creosote for photography. Measurements were made using ImageJ software (Wayne Rasband, NIH, USA) from photographs taken on an Olympus BX51 compound microscope mounted with DP25 camera attachment (Olympus, Tokyo). All measurements are in micrometres unless otherwise indicated, and in descriptions are given as range, followed by mean in parentheses, where numbers permit. To compare overall size between sexes a two-tailed Student’s t-test was computed in Excel (Microsoft, 2017). Drawings were made from photographic series.
Specimens chosen for scanning electron microscopy (SEM) were transferred to 2.5 % gluteraldehyde in 0.1 M phosphate buffer, post-fixed in 1% osmium tetroxide and dehydrated through a gradient series of ethanols, critical-point dried in a CPD030 BalTec critical-point dryer (BalTec AG, Balzers, Liechtenstein) using carbon dioxide, mounted on aluminium stubs, and sputter coated with gold/palladium (60:40) to a thickness of 10 nm in an Emitech K575X Peltier-cooled high-resolution sputter coater (EM Technologies, Ashford, Kent, UK). The specimens were viewed with a JEOL 6700 F field emission scanning electron microscope (JEOL Ltd., Tokyo, Japan) at the Otago Centre for Electron Microscopy (OCEM, University of Otago, New Zealand).
Molecular data and analyses
Genomic DNA from one individual was extracted using the DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. A partial fragment of 28S rRNA gene was amplified using T16 and T30 primers (Harper & Saunders, 2001) and conditions following Bennett et al. (2023). PCR products were cleaned using EXOSAP Express PCR Product Cleanup Reagent (USB Corporation, Cleveland, OH, USA), following manufacturer’s instructions. Sanger sequencing by capillary electrophoresis was performed by the Genetic Analysis Service, Department of Anatomy, University of Otago (Dunedin, New Zealand).
The successfully amplified sequence was imported into Geneious Prime®v1.2, trimmed using the trim function with default parameters, and manually edited for incorrect or ambiguous base calls. An alignment was created with the new sequence and other closely related species and representatives from genus Polymorphus. Uncorrected pairwise genetic divergences within genus and between species were calculated in MEGA v.11 and the resulting 28S sequence was deposited with GenBank under accession OR593504.
Results
A total of 50 acanthocephalans was found in 4 birds. Using the key to the genera of the Polymorphidae of Presswell et al. (2020), the specimens clearly key out to genus Polymorphus. A consideration of the numbers of hook rows and number of hooks per row, size of adult worms, presence or absence of sexual dimorphism, egg size, genetic sequences and geographical locality found no nominal species comparable to the New Zealand specimens, and they were adjudged to represent a species new to science, which is described below.
ACANTHOCEPHALA Koehlreuther, 1771
Polymorphida Petrochenko, 1956
Polymorphidae Meyer, 1931
Polymorphus Lühe, 1911
Polymorphus circi n. sp. (Figures 1, 2 and 3)
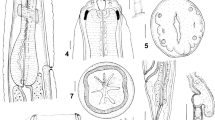
Polymorphus circi n. sp. scanning electron micrographs. (a) praesoma showing distribution of trunk spines, (b) proboscis, (c) higher magnification of trunk spines, (d) cross-section of male through cement glands (numbered 1 to 4), (e) Eggs. Scale bars: a = 1 mm; b and d= 100 µm; c = 50 µm; e = 20 µm.
General [based on 25 specimens]: With characters of the genus. Cream in colour when removed from intestine. No significant sexual size dimorphism (two–tailed t–test, t(17) = -1.128, P>.05 based on mean length of adult worms). Trunk cylindrical, with anterior expansion for approximately one quarter of worm length in both sexes. Anterior expansion with single field of spines in 23–25 circles; spinous field equally long dorsally and ventrally; spines more or less uniform in size, 44–55 long, and evenly spaced in a fully extended specimen. Hypodermal nuclei extend from about middle of trunk spine field to near the posterior end of trunk. Neck conical, broadest at base, about two thirds as long as proboscis in fully extended specimens. Proboscis cylindrical, with 20–22 rows of 11–13 hooks; hooks more or less equal in size throughout, but posteriormost two or three finer. Roots simple, longer than blades in anterior hooks, small with anteriorly directed manubria in posteriormost 2 or 3 hooks. Proboscis receptacle inserted at base of proboscis, double walled, with cerebral ganglion at mid–point. Lemnisci clavate, longer than proboscis receptacle.
Adult male [based on 11 specimens]: Trunk 8.20–9.93 (9.21) mm long by 0.97–1.59 (1.20) mm at widest part of expansion. Spine field 1.20–1.96 mm long. Proboscis 544–676 (611) long by 196–235 (210) wide at middle; 20–22 longitudinal rows of 11–13 hooks each; hook lengths [root lengths] in 1 male measured from proboscis anterior, 36 [–], 38 [39], 40 [43], 40 [43], 36 [45], 41 [43], 42 [51], 38 [–], 39 [–], 40 [17], 27 [12]. Neck 240–401 (342) long. Proboscis receptacle 949–1,184 (1090) long by 199–304 (251) wide. Lemnisci 1,089–1,579 (1,345) long by 214–356 (299) wide posteriorly. Testes ovoid, oblique, overlapping, close to posterior end of lemnisci, sometimes overlapping lemnisci; anterior testis 505–864 (744) long by 316–506 (419) wide, posterior testis 510–1,016 (741) long by 339–513 (442) wide. Cement glands 4 (Fig. 2d), tubular, unequal lengths, reaching as far as posterior testis; longest 3,454–6,145 (4,875) long by 180–247 (210) wide. Saefftigen’s pouch 922–1244 (1022) long. Posterior extremity rounded, with terminal genital pore. Copulatory bursa with many sensory pits, length 667–865, width 749–1194 (n=3) (Fig. 3c), size dependent upon stage of eversion.
Adult female [based on 8 specimens]: Trunk 7.78–9.99 (8.89) mm long by 1.13–1.48 (1.30) mm at widest part of expansion. Spine field 1.42–1.86 mm long. Proboscis 556–821 (670) long by 210–232 (221) wide at middle, 20–22 longitudinal rows of 10–13 hooks each; hook lengths [root lengths] in 1 female measured from proboscis anterior, 31 [36], 42 [45], 42 [44], 45 [50], 41 [49], 38 [45], 40 [39], 36 [36], 35 [21], 30 [20], 31 [–]. Neck 363–403 (383) long. Proboscis receptacle 911–1,267 (1,045) long by 211–298 (264) wide. Lemnisci 1,336–1,801 (1,574) long by 308–324 (316) wide posteriorly. Posterior extremity rounded, sometimes slightly inflated on ventral aspect; genital pore sub-terminal, on ventral surface (Fig. 3d). Female reproductive tract difficult to observe, obscured by eggs in older specimens. In one female, of 8.2 mm long, entire reproductive system 3.5 mm long, vaginal complex 690 long. Eggs elongate with polar prolongation of middle membrane, outer shell 80–111 (89) long by 21–27 (23) wide.
Immature male [based on 2 specimens]: Specimen with reproductive organs present, but in early development, proboscis and bursa inverted. Trunk 2.35–2.36 mm long, 526–535 wide. Proboscis (inverted) 409 long (n=1); proboscis receptacle 525–716 long, 159–189 wide. Testes small and almost parallel. Anterior testis 322 long, 125 wide; posterior testis 307 long, 148 wide (n=1).
Immature female [based on 1 specimen]: Specimen with no visible signs of reproductive tract, ovarian balls or eggs. Trunk 2.57 mm long, 546 wide. Proboscis (part inverted) 486 long, 192 wide; proboscis receptacle 812 long, 189 wide. Lemnisci folded and crumpled to appear shorter than proboscis receptacle.
Type host. Australasian harrier Circus approximans Peale (Accipitriformes: Accipitridae).
Type locality. Timaru, Canterbury District, South Island, New Zealand. 44°23′54″S, 171°15′36″E.
Other localities. Maungatua and Waihola, Taeiri plain, Dunedin, South Island, NZ. 45°55', 170°09'.
Site of infection. Intestine.
Prevalence and intensity. In four birds out of 65 (6%), intensity 1 to 40 (mean 12.5)
Type material. Holotype male W.003957 Allotype female W.003958 Paratypes, 5 specimens W.003959 from Taieri Plain: deposited with the Museum of New Zealand, Te Papa Tongarewa, Wellington, NZ.
Voucher material. Hologenophore W.003960
Representative DNA sequence. Genbank accession OR593504
Zoobank taxon reference. 43CC3935-AECB-47D9-B06C-D94C5BE57E6E
Etymology. The specific name “circi” (a masculine noun in genitive case) relates to the genus of the unusual definitive host.
Remarks
This polymorphid acanthocephalan exhibits a number of characters that define it as a species of Polymorphus: trunk cylindrical with anterior expansion, foretrunk with a single field of spines, proboscis cylindrical to ovoid, hooks slightly larger in middle, proboscis receptacle double-walled and inserted at base of proboscis, ganglion near middle of receptacle, lemnisci clavate, testes in anterior half of trunk, four tubular cement glands, eggs with polar prolongation of middle membrane (Amin 1992; Presswell et al. 2020).
Despite considerable historical disagreement over the members of this genus, 36 species of Polymorphus are currently recognised in the literature (Amin 2013). Of these nearly all are reported from ducks, waders and other freshwater and marine birds. Four species are recorded from accipitriform birds: P. brevis (van Cleave, 1916) Travassos 1926, P. magnus Skrjabin, 1913, P. meyeri Lundström, 1942 and P. striatus (Goeze, 1782) Lühe 1911. Each of these records is however supplemental to more frequent reports of aquatic bird hosts, and three of the records involve Haliaeetus spp. which are fish-eagles. Only one record involves a truly terrestrial raptor host; P. magnus, which was found in the tawny eagle Aquila rapax (Temminck), an accipitrid with a scavenging habit similar to Circus approximans (Ferguson-Lees & Christie 2020). Of those species found elsewhere in raptors, none shares the same hook arrangement as P. circi n. sp. (20–22 rows of 11–13 hooks): P. brevis has 18 rows of 15 hooks, P. magnus has 14–18 rows of 7–9 hooks, P. meyeri has 16–17 rows of 6 hooks, and P. striatus has 16 rows of 12–16 hooks.
Only one species has been recorded from the nearest landmass, Australia: P. biziurae Johnston & Edmonds, 1948, found in a musk duck Biziura lobata (Shaw); it has a similar hook arrangement (21–22 rows of 9–11 hooks) to P. circi n. sp., but differs in its sexual size dimorphism, greater trunk length of the female (1.7 mm–18.2 mm), greater length of the proboscis receptacle (1.2–2.3 mm) and smaller size of the eggs, which lack polar prolongations.
All of the other nominal species differ from P. circi n. sp. in their proboscis hook arrangement, except for P. cucullatus van Cleave & Starrett, 1940, P. mathevossianae Petrochenko, 1949, P. spindlatus Amin & Heckmann, 1991, and P. swartzi Schmidt, 1965. Polymorphus cucullatus (22 rows of 12 hooks) is a larger worm (males 11-13 mm, females 10 mm) with a pyriform proboscis rather than cylindrical, and much larger proboscis hooks (maximum length 85 µm as opposed to 51 µm in P. circi n. sp., P. mathevossianae (20 rows of 11–12 hooks) is much smaller in size (males and females 2.6–4.5mm) and has a very long field of trunk spines (60 circles), P. spindlatus (18–20 rows of 11–13 hooks) has a distinctive spindle-shaped proboscis and is much smaller in size (males 3.5–5.2 mm, females 3.6–6.0 mm), and P. swartzi has a swollen proboscis and is also smaller (female only described, 4.7 mm).
Genetic results
Our newly generated 28S sequence of Polymorphus circi n. sp. was closest to Polymorphus trochus from an American coot Fulica americana GenBank accession JX442185 (García-Varela et al. 2013) with 37.1% genetic divergence at 28S. The average genetic divergence observed between species within Polymorphus was 41.0%, ranging between 34.2-51.5%. This comparison included available representatives from GenBank, including Polymorphus brevis AY829105 and JX442183, P. trochus JX442185, P. minutus EU267819, P. obtusus JX442184 and Polymorphus sp. AY829109. These large divergences illustrate the considerable variability in the 28S gene for these acanthocephalans. For instance, García-Varela et al. (2013) used Pro-Align to detect and remove unreliably aligned sections from their polymorphid 28S alignment, which resulted in a 26% loss of sites. Because the sequences were essentially unalignable, any phylogenetic inferences were unreliable, so a tree is not presented here. Amplification of three other genes (18S, ITS and cox1) was unfortunately unsuccessful.
Discussion
No named species of Polymorphus has been recorded in New Zealand previously, but there are two reports of unidentified species. McDonald (1998) noted the presence of Polymorphus sp. in a pied stilt Himantopus leucocephalus Gould. However, McDonald’s specimens were initially identified as belonging to genus Falsifilicollis, which, following a tortuous history of synonymisation and subsumption, is now regarded as Profilicollis (García-Varela & Pérez-Ponce de León 2008). Smales et al. (2010) also recognised the stilt record as Profilicollis sp. A second report of Polymorphus sp. in New Zealand was of an acanthella (pre-encysted juvenile stage) from a hedgehog Erinaceus europaeus L. (Smales et al. 2010). All paratenic hosts of acanthocephalans are vertebrates (Kennedy 2006), and hedgehogs have been recorded as paratenic hosts for a related acanthocephalan, Plagiorhynchus cylindraceus (Goeze, 1782), both in New Zealand and in their native Europe (Skuballa et al. 2010). Plagiorhynchus cylindraceus has a non-aquatic life cycle that includes terrestrial isopods as intermediate hosts and passerine or corvine birds as definitive hosts (Skuballa et al. 2010), so infection of a bird scavenging hedgehog carcases is highly plausible. Polymorphus species, on the other hand, are only known to exhibit an aquatic life cycle, thus making infection of a hedgehog less probable. The finding of Smales et al. (2010) was the only occurrence on record, so this is a presumably a rare phenomenon. The Polymorphus sp. specimen in question possessed 20 rows of 5–6 hooks, thus was not the same species as P. circi n. sp.
The terrestrial lifestyle of the harrier raises the question of a potential intermediate host for Polymorphus circi n. sp. The intermediate host of Polymorphus species is always a crustacean; most usually an amphipod or occasionally a crayfish (Crompton and Nickol 1985). If the life cycle of this new species is completed in freshwater, the only crustacean large enough to count as prey would be the native crayfish, Paranephrops zealandicus (White), found on Stewart Island and on the eastern side of South Island (Whitmore et al. 2000). Freshwater crayfish are not unknown as intermediate hosts for Polymorphus species: P. biziurae Johnston & Edmonds, 1948 (parasite of the musk duck Biziura lobata in Australia) and P. boschadis (Schrank, 1788) [now P. minutus] (parasite of Anatidae in Europe) have been found in Cherax destructor (Clark) (Edgerton et al. 2002). If the life cycle of this species is completed in marine water, the intermediate host would likely be an amphipod. Although there are records of harriers feeding on crabs in intertidal areas (Latham 2002; Totterman 1997) they are unlikely to be intermediate hosts for this acanthocephalan. Nickol et al. (1999) reviewed the morphology of all species assigned to Polymorphus that were found as cystacanths in crabs and concluded “it appears that all known records of Polymorphus in decapods refer to species of the subgenus Profilicollis...” (Nickol et al. 1999), thereby ruling out crabs as potential hosts of Polymorphus. The most likely contender for intermediate host seems to be terrestrial amphipods (Talitridae). There are 28 species of talitrid in New Zealand; they are found in damp habitats and make up a significant component of the leaf litter and soil fauna (Fenwick & Webber 2008). Unfortunately, the origin of our infected birds does nothing to clarify the question of intermediate host: of the three birds from a known locality, two were found on the Taeri Plain (an inland low-lying flood plain with rivers, lakes and marshland) and one was found almost adjacent to the marine water at the port of Timaru. Further examination of likely arthropod contenders from relevant areas is ongoing.
The literature review required for this study highlighted some discrepancies in current species descriptions. Four species from Pakistan are incertae sedis as no trunk spines are recorded in their descriptions (P. fatimaae Khan, Dharejo, Birmani and Bilqees, 2008, P. mohiuddini Muti-ur-Rahman, Khan, Bilqees and Khatoon, 2008, P. nickoli Khan and Bilqees, 1998 and P. sindhensis Khan, Ghazi and Bilqees, 2002). We have not seen examples of these species so refrain from reallocating them, but a number of features in the descriptions suggest them to be species of Centrorhynchus. These specimens require re-examination to place them in the correct genus.
There are many reports in the literature of acanthocephalans causing pathological symptoms or death to their hosts. The proboscis burrows into the intestinal wall where it is secured by the proboscis hooks, in order to hold the parasite in its preferred site in the gut. The lesions thus caused may affect innervation of the intestine, as well as secretory and motor function (Petrochenko 1958). In some cases, the whole worm may burrow right through the intestine and come to rest in the body cavity resulting in potentially lethal peritonitis (Clark et al. 1958). In very severe infections, or when birds are otherwise health-compromised, Polymorphus spp. have been implicated in mass fatalities among bird hosts (Crompton and Nickol 1985). Although none of the infections found in harriers was sufficient to have been the cause of death, in the light of the potential for pathogenicity provided by these acanthocephalans it is advised that conservation and animal health practitioners in New Zealand are alert to the occurrence of Polymorphus circi n. sp. in harriers or other potential hosts.
Data Availability
Not applicable.
Code availability
Not applicable.
References
Amin, O. M. (1992). Review of the genus Polymorphus Lühe, 1911 (Acanthocephala: Polymorphidae), with the synonymization of Hexaglandula Petrochenko, 1950, and Subcorynosoma Hoklova, 1967, and a key to the species. Qatar University Science Journal, 12, 115–123. http://hdl.handle.net/10576/9865
Amin, O. M. (2013). Classification of the Acanthocephala. Folia Parasitologica, 60, 273–305. https://doi.org/10.14411/fp.2013.031
Baker-Gabb, D. J. (1981). The diet of the Australasian harrier (Circus approximans) in the Manawatu–Rangitikei sand country, New Zealand. Notornis, 28, 241–254.
Bennett. J., Presswell. B., and Poulin. R. (2023). Tracking life cycles of parasites across a broad taxonomic scale in a marine ecosystem. International Journal for Parasitology 53: 258–303. https://doi.org/10.1016/j.ijpara.2023.02.004
Clark, G.M., O'Meara, D., and Van Weelden, J.W. (1958). An epizootic among eider ducks involving an acanthocephalid worm. Journal of Wildlife Management 22: 204–205.
Crompton, D.W.T., and Nickol B.B. (1985). Biology of the Acanthocephala. Cambridge University Press, Cambridge, 519 pp.
Debus., S., and Kirwan, D. M. (2020). Swamp Harrier (Circus approximans), version 1.0. In Birds of the World (J. del Hoyo, A. Elliott, J. Sargatal, D. A. Christie, and E. de Juana, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.swahar1.01
Eakle, W. L. (2008). Relative abundance of Australasian harriers (Circus approximans) in New Zealand. Notornis, 55, 136–139.
Edgerton, B. F., Evans, L. H., Stephens, F. J., and Overstreet, R. M. (2002). Synopsis of freshwater crayfish diseases and commensal organisms. Aquaculture, 206(1–2), 57–135. https://doi.org/10.1016/S0044-8486(01)00865-1
Fenwick, G., and Webber, R. (2008). Identification of New Zealand’s terrestrial amphipods (crustacea: amphipoda: talitridae). Tuhinga: Records of the Museum of New Zealand Te Papa Tongarewa, 19, 29–56.
Ferguson-Lees, J. and Christie, D. (2020). Raptors of the World. Bloomsbury. E-book 320 pp.
García-Varela, M., and Pérez-Ponce de León, G. (2008). Validating the systematic position of Profilicollis Meyer, 1931 and Hexaglandula Petrochenko, 1950 (Acanthocephala: Polymorphidae) using cytochrome c oxidase (cox1). Journal of Parasitology, 94, 212–218. https://doi.org/10.1645/GE-1257.1
García-Varela, M., Pérez-Ponce de León, G., Aznar, F. J., and Nadler, S. A. (2013). Phylogenetic relationship among genera of Polymorphidae (Acanthocephala), inferred from nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution, 68, 176–184. https://doi.org/10.1016/j.ympev.2013.03.029
García-Varela, M., Pérez-Ponce de León,, G., Aznar, F. J., and Nadler, S. A. (2011). Erection of Ibirhynchus gen. nov. (Acanthocephala: Polymorphidae), based on molecular and morphological data. Journal of Parasitology, 97, 97–105. https://doi.org/10.1645/GE-2350.1
Harper, J. T. and Saunders, G. W. (2001). Molecular systematics of the Florideophyceae (Rhodophyta) using nuclear large and small subunit rDNA sequence data. Journal of Phycology 37, 1073–1082. https://doi.org/10.1046/j.1529-8817.2001.00160.x
Johnston, T. H., and Edmonds, S. J. (1948). Australian Acanthocephala No. 7. Transactions of the Royal Society of South Australia, 72(1), 69–76.
Kennedy, C. R. (2006). Ecology of the Acanthocephala. Cambridge University Press. 249 pp. https://doi.org/10.1017/CBO9780511541902
Latham, A. D. M. (2002). Australasian harrier (Circus approximans) observed feeding on crabs at Hooper’s Inlet, Otago Peninsula. Notornis, 49(1), 53–54.
McDonald, S. (1998). The parasitology of the black stilt (Himantopus novaeazelandiae). A report submitted to the Department of Conservation Threatened Species Unit. 62 pp.
Nickol, B. B., Crompton, D. W. T., and Searle, D. W. (1999). Reintroduction of Profilicollis Meyer, 1931, as a genus in Acanthocephala: significance of the intermediate host. The Journal of Parasitology, 85(4), 716–718. https://doi.org/10.2307/3285748
Petrochenko, V. I. (1958). [Acanthocephala of domestic and wild animals.] Vol. 2. Moscow: Izdatel’stvo Akademii Nauk SSSR. (In Russian; English translation by Israel Program for Scientific Translations, Ltd., Jerusalem, 1971). 478 pp.
Presswell, B., Bennett, J. D., and Smales, L. R. (2020). Morphological and molecular characterisation of a new genus and species of acanthocephalan, Tenuisoma tarapungi n. g., n. sp. (Acanthocephala: Polymorphidae) infecting red–billed gulls in New Zealand, with a key to the genera of the Polymorphidae Meyer, 1931. Systematic Parasitology, 97, 25–39. https://doi.org/10.1007/s11230-019-09898-0
Robertson, H.A., Baird, K.A., Elliott, G., Hitchmough, R., McArthur, N., Makan, T., Miskelly, C., O’Donnell, C.F., Sagar, P.M., Scofield, R.P. and Michel, P. (2021). Conservation status of birds in Aotearoa New Zealand, 2021. Department of Conservation, Te Papa Atawhai.
Sadleir, R. M., and Linklater, W. L. (2016). Annual and seasonal patterns in wildlife road-kill and their relationship with traffic density. New Zealand journal of Zoology, 43(3), 275–291. https://doi.org/10.1080/03014223.2016.1155465
Skuballa, J., Taraschewski, H., Petney, T. N., Pfäffle, M., and Smales, L. R. (2010). The avian acanthocephalan Plagiorhynchus cylindraceus (Palaeacanthocephala) parasitizing the European hedgehog (Erinaceus europaeus) in Europe and New Zealand. Parasitology Research, 106, 431–437. https://doi.org/10.1007/s00436-009-1681-9
Smales, L. (2003). An annotated checklist of Australian Acanthocephalan from mammalian and bird hosts. Records of the South Australian Museum, 36(1), 59–82.
Smales, L., Skuballa, J., Taraschewski, H., Petney, T. and Pfäffle, M. (2010). An immature polymorphid acanthocephalan from a European hedgehog (Erinaceidae) from New Zealand. New Zealand Journal of Zoology, 37(2), 185–188. https://doi.org/10.1080/03014223.2010.488788
Totterman, B. G. (1997). Swamp harriers preying on crabs. Australian Bird Watcher, 17(2), 101.
Whitmore, N., Huryn, A. D., Arbuckle, C. J., and Jansma, F. (2000). Ecology and distribution of the freshwater crayfish Paranephrops zealandicus in Otago. Science for Conservation, 148, 5–42.
Yamaguti, S. (1963). Systema Helminthum. Vol. V. Acanthocephala. London: Interscience Publishers, 423 pp.
Acknowledgements
The authors would like to thank the Dunedin Wildlife Hospital, Clement Lagrue at Department of Conservation, and Jenni Fraser at the NZ Raptor Trust for providing harrier carcases for dissection. We thank and acknowledge mana whenua and local rūnanga (Te Rūnanga o Ōtākou, Te Rūnanga o Moeraki and Kāti Huirapa Rūnanga ki Puketeraki) for their support of our disease screening process of Otago birds. We are grateful for the assistance of the electron microscopy staff at Otago Micro and Nanoscale Imaging at the University of Otago. We thank an anonymous reviewer for appreciating this work without demur. And, as always, thank you to Robert Poulin for his continued support of New Zealand parasite taxonomy and his comments on this paper.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was partially funded by a Birds New Zealand Research Fund Grant. JB was supported through the Cawthron Institute’s Ministry of Business, Innovation and Employment, Emerging Aquatic Diseases Endeavour Grant, Award Number CAWX2207.
Author information
Authors and Affiliations
Contributions
Both authors were responsible for designing the study and for dissection and processing of birds. BP did taxonomic research, morphological study, and figures. JB performed all molecular techniques and sequencing. Both authors were responsible for SEM imaging. Both authors wrote the remaining text and agreed on the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Receipt and handling of dead birds in this study complies with a Department of Conservation permit 65658-DOA awarded to the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Presswell, B., Bennett, J. Description and molecular data for a new acanthocephalan parasite, Polymorphus circi n. sp. (Polymorphidae) from the Australasian harrier (Circus approximans Peale) in New Zealand. Syst Parasitol 100, 725–733 (2023). https://doi.org/10.1007/s11230-023-10120-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-023-10120-5